New Ferulic Acid Derivatives Protect Against Carbon Tetrachloride-Induced Liver Injury in Rats
Abstract
Background:
Ferulic Acid (FA) is a phenolic compound that exhibits a wide range of therapeutic effects majorly due to its antioxidant property. There have been recent efforts to maximize the therapeutic value of FA by way of structural modification of FA to form FA derivatives in order to enhance physicochemical, antioxidant and therapeutic prospects.
Objective:
In this work, we synthesized, characterized and evaluated new ferulic derivatives [ferulic phthalate and maleate] for their potential to protect against CCl4-induced liver injury in Wistar rats.
Methods:
Male Wistar rats were randomly assigned into groups and given oral administration of CCl4 only, or in combination with either ferulic phthalate or maleate and thereafter, some biochemical parameters (alanine transaminase, aspartate transaminase, alkaline phosphatase and malondialdehyde) and antioxidant indices (superoxide dismutase, reduced glutathione) were determined.
Results:
Exposure to CCl4 altered liver biochemical indices in particular plasma alanine transaminase activities, in a manner reminiscent of liver injury. However, ferulic acid, as well as the ferulic derivatives significantly (p<0.05), improved rat liver indices. Also, ferulic derivatives improved (p<0.05) the rat antioxidant status compared with the group given CCl4 only. Furthermore, ferulic acid and ferulic maleate significantly (p<0.05) ameliorated rat lipid peroxidation caused by CCl4. Histological studies revealed mild infiltration caused by CCl4 but this cellular distortion was abated and improved in rats administered with ferulic acid, ferulic phthalate as well as ferulic maleate.
Conclusion:
These findings do not only support the antioxidant as well as the therapeutic potentials of ferulic phthalate and maleate but also the medicinal value and prospects of phenolic compounds for therapeutic purposes.
1. INTRODUCTION
Ferulic Acid (FA) is a phenolic compound with important antioxidant potential. It is a precursor to many other valuable phenolics [1, 2]. It is a ubiquitous phenolic plant constituent that exhibits a wide range of therapeutic effects against cancer, diabetes, cardiovascular and neurodegenerative diseases, on account of its potent antioxidant capacity [3]. FA is one of the most abundant phenolic acids found in plants, many staple foods such as fruits, vegetables, cereals, and coffee [4, 5]. The antioxidant property of FA may be due to the ability of its hydroxy and phenoxy groups to donate electrons to quench the free radicals. For example, the nucleophilic groups on the benzene ring (3-methoxy and more importantly, 4- hydroxyl) of FA give the additional property of terminating free radical chain reactions. Another functional group in the FA, i.e. the carboxylic acid group with an adjacent unsaturated C-C double bond can provide additional attack sites for free radicals and thus prevent them from attacking the membrane. In addition, this carboxylic acid group also binds to the lipid bilayer, providing some protection against lipid peroxidation [6].
There have been increasing efforts to maximize the therapeutic benefits of FA. Among such efforts is the structural modification of FA to form FA derivatives with enhanced physicochemical and antioxidant properties [7-9]. Indeed, studies have implicated several FA derivatives for therapeutic potentials, such as anticancer [10-12], antiatherogenic [13], and antibacterial agents [14], as well as anti-inflammatory activity [15, 16]. Furthermore, the esterification of FA has also been widely used to increase its biological potency [17]. FA ethyl ester is derived by the addition of ethyl groups to FA by the esterification reaction between FA and ethanol [18]. The incorporation of these ethyl groups makes it more lipophilic, increasing the ability of this derivative to cross lipid-rich cell membranes, thereby offering better antioxidant potential [19], as the membranes such as the blood-brain barrier become more permeable and accessible. Also, FA methyl esters have been shown to be highly lipophilic possessing greater antioxidant activity than the parent compound FA [20, 21]. Isoferulic acid is another derivative of FA which has been implicated for anti-diabetic potential [22, 23]. In addition, alkyl ferulates such as 1-hexyl ferulate, 1-heptyl ferulate and 1-pentyl ferulate, have been demonstrated as possessing a higher inhibitory effect on linoleic acid peroxidation [8], while some synthesized derivatives have been observed to improve the stability and absorptions [24].
In the present study, we synthesized and characterized new FA derivatives and also evaluated them for potential protection against CCl4-induced liver injury in Wistar rats.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
All chemicals and reagents used were of analytical grade and sourced from Sigma-Aldrich (St Louis, MO, USA). Com-mercial reagent kits for the assay of Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were as supplied by Randox Diagnostics, Crumlin, UK. Ferulic acid was obtained from Sigma Aldrich, China (128708-100G Lot # STB-C5005V). Where necessary, solvents were redistilled prior to use. Thin Layer Chromatography (TLC) was performed using pre-coated silica gel 0.25 mm 60 F254 plates (Merck, Germany) and developed plates were viewed under the UV light (254 and 365 nm).
2.2. Synthesis of Ferulic Phthalate (FP) and Ferulic Maleate (FM)
The ferulic acid derivatives were synthesized following standard procedure [25]. Ferulic acid (5 mmol, 194 g) was dissolved in 5% NaOH, then 4.44 g of phthalic anhydride (5mmol, 148 g) or 1.47 g Maleate anhydride (5mmol, 98 g) was added gently to the aqueous solution and stirred on ice for 30 minutes. The reaction process was allowed to progress with constant monitoring on a Thin Layer Chromatograph (TLC) plate to completion. The product was purified by washing with cold saturated brine solution dried at room temperature and stored for further analysis.
2.2.1. Characterization of Synthesized Ferulic Derivatives
Melting points were determined on an Electrothermal, UK, Melting point apparatus. Absorption data was obtained on Beckman Coulter DU 730 Life Sciences, UK, UV-Visible spectrophotometer while infrared spectra were obtained on a Shimadzu (8400S) Fourier Transform-Infrared using KBr pellet over a 500-4000 cm-1 range. The molecular weight was determined on Mass Spectrometry MS-QP 2010 PLUS (Shimadzu Japan) system coupled with a finigan MAT ion trap detector operating with Electron Impact (EI) Ionization mode at a voltage of 70 eV.
2.3. Experimental Animals and Treatment
Thirty-five (35) male Wistar rats weighing between 120 and 150 g were randomly assigned into five groups. The animals were acclimatized to housing conditions of temperature and relative humidity, with a 12 h light/12 h dark cycle. Water and pellets were provided ad libitum. Group 1 served as positive control and received carbon tetrachloride (CCl4; 1ml/ kg bw) alone; group 2 served as negative control and received distilled water only (vehicle; 1ml/ kg bw); while groups 3, 4, 5, 6 and 7 in addition to carbon tetrachloride (1ml/ kg bw) given on days 1, 3 and 7, received ferulic acid (40 mg/kg bw), ferulic phthalate or ferulic maleate at 3 or 7 mg/kg bw respectively for 14 days.
The CCl4 dose (1/2 LD50 – 1 ml/kg bw) was selected based on a previous study reported elsewhere [26], while the dose selection for ferulic acid was based on a report by Balasubashini et al. [27] which demonstrated that oral administration of ferulic acid at 40 mg/kg bw was safe in rats. The treatments were daily by oral gavage and lasted for 14 days. Handling of animals was in accordance with Landmark University ethical guidelines as approved for scientific study, and consistent with relevant guidelines on the care and use of laboratory animals [28].
2.3.1. Preparation of Plasma and Liver Homogenates
The rats were sacrificed after 14 days of treatment. Blood sample was collected in heparinized bottles and centrifuged at 5000 g (Anke TDL-5000B, China) to obtain the plasma. The liver was collected and homogenized in ice-cold isotonic solution (0.25 M sucrose) and further centrifuged to obtain the supernatant used for biochemical assays.
2.4. Biochemical Assays
The biochemical parameters were determined in rat plasma using a UV/Vis spectrophotometer (Jenway 7205, Staffordshire, United Kingdom) where applicable. The concentrations of rat liver and plasma aspartate aminotransferase (AST – EC: 2.6.1.1), alanine aminotransferase (ALT – EC 2.6.1.2), were determined using the Randox assay kits (Crumlin, UK). Alkaline phosphatase (ALP – EC: 3.1.3.1) was determined using the method described by Wright et al. [29]. Total protein was determined using the Lowry’s method [30]. Malondialdehyde (MDA) was determined using the method described by Niehaus and Samuelson [31]. Reduced Glutathione level (GSH) was determined using the procedure described by Ellman [32], and Akanji et al. [33]. Superoxide dismutase (SOD – EC 1.15.1.1) was determined using the method described by Misra and Fridovich [34].
2.5. Histopathological Examination
Staining for liver histology was described elsewhere [35]. Briefly, rat liver was fixed in 10% buffered neutral formalin immediately following excision from animals. The tissue fixation and subsequent histopathological processes including capture and scoring for morphological changes were done by a pathologist blind to the treatments, at the University of Ilorin Teaching Hospital, Ilorin, Nigeria. The photomicrographs were captured using the software, Presto! Image Folio package (×400 magnification).
2.6. Data Analysis
Data were analyzed on GraphPad Prism 5 (GraphPad Software Inc. San Diego, California) using one-way analysis of variance (ANOVA). Post-hoc-test were conducted with the Dunnett’s multiple comparison test. Data are recorded as means of replicates ± Standard Error of Mean (SEM). Group mean values at p˂ 0.05 were considered significant.
3. RESULTS
3.1. Synthesis and Characterization of Ferulic Maleate and Ferulic Phthalate
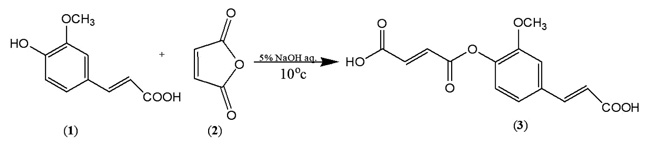
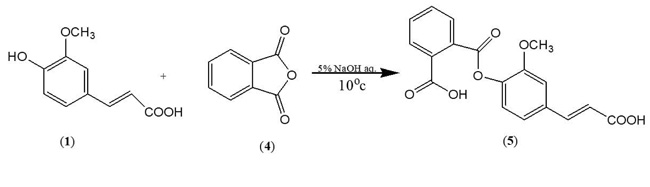
In order to synthesize a bioactive ferulic maleate (Scheme A) and ferulic phthalate (Scheme B) adducts, ferulic acid (1) was treated with maleate anhydride (2) in aqueous sodium hydroxide solution in the absence of a protecting group on the carboxylic acid. A similar treatment was given to 1 using phthalic acid (4) to obtain (5). The resulting ferulic maleate (3) and ferulic phthalate (5) was purified by washing with brine and purified product thereafter analyzed using the melting point apparatus, Fourier transform infrared and mass spectrometry.
3.1.1. Ferulic Maleate (3)
White powder, m.p. 140-142 oC, yield 72%, (Rf = 0.92, DCM), (UV/Vis = 323 nm, 2.7 abs), IR (KBr) (Vmax, cm-1) 3431, 2933, 1685, 1431, 1273, 1209, 1035, 943, 862 and 790, ; C14H12O7; MW=292.24 MS: m/z (%): 206, 150, 135, 118, 107.
3.1.2. Ferulic Phthalate (5)
White powder, m.p. 183-184 oC, yield 72%, (Rf =0.76, DCM), (λ max =321 nm, 2.7abs), IR (KBr) (Vmax, cm-1) 3437,, 3014, 1693, 1593, 1514, 1406, 1278, 1207; C18H14O7; MW=342.3; MS: m/z (%) [M-1]+ 341. Other fragment ions: 327, 281, 207, 150, 135.
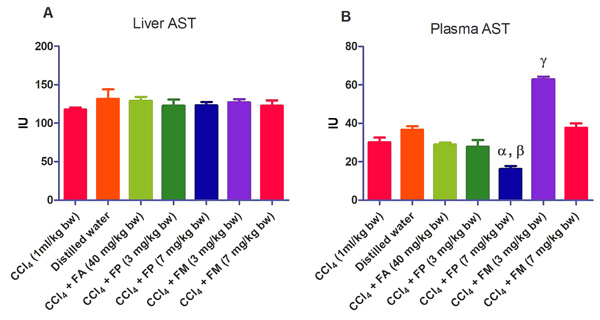
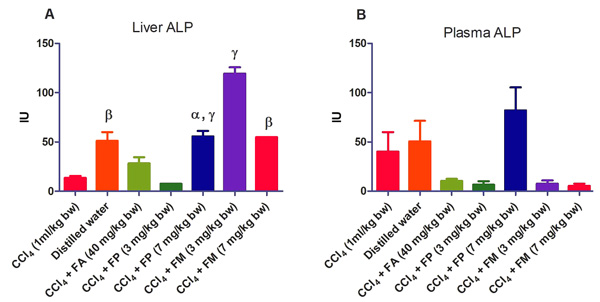
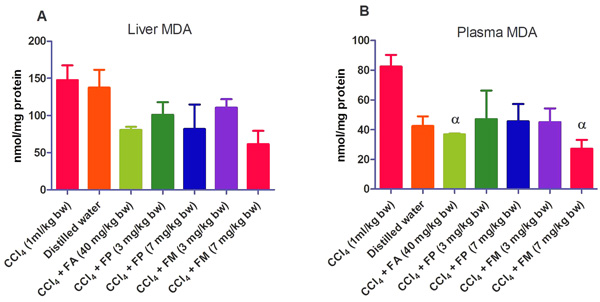
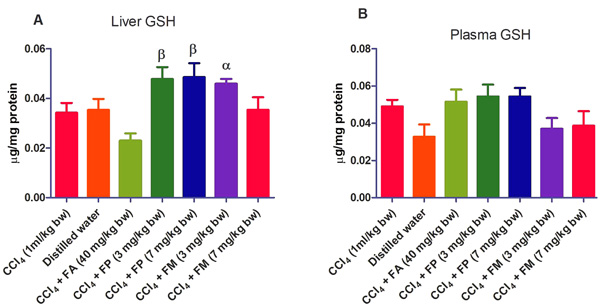

3.2. Biochemical Assays
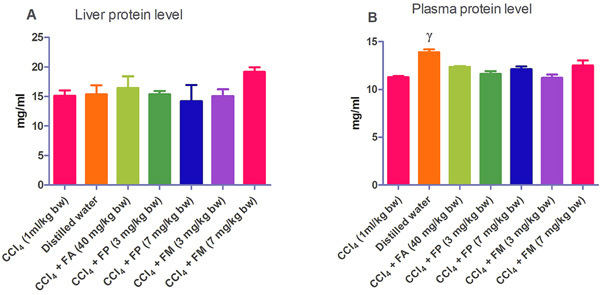
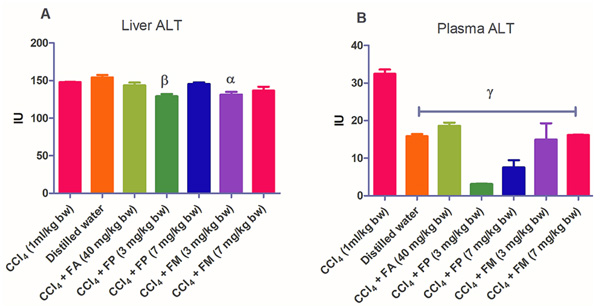
Administration of CCl4 alone significantly decreased plasma protein concentration. But the administration of CCl4 did not alter the rat liver protein concentration compared with the other treatment groups (Fig. 1a-b). ALT levels in rat liver of the groups co-administered with either ferulic phthalate or maleate at 3 or 7 mg/kg bw were significantly (p<0.05) lowered than that of the CCl4 group (Fig. 2a). However, there was no corresponding increase in the plasma of these same treatment groups as the ferulic acid and its derivatives, significantly (p<0.05) ameliorated the increase in rat plasma level of ALT caused by CCl4 (Fig. 2b). Administration of CCl4 alone did not significantly affect the rat liver AST level relative to the other treatment groups (Fig. 3a). However, relative to the CCl4 group, co-treatment with either ferulic phthalate (7 mg/kg bw) or ferulic maleate (3 mg/kg bw) significantly altered the rat plasma level of AST (Fig. 3b). Administration of CCl4 alone significantly (p<0.05) reduced ALP level in rat liver (Fig. 4a). However, the co-administration of CCl4 and ferulic maleate (3 and 7 mg/kg bw) or ferulic phthalate (7 mg/kg bw) significantly increased rat liver ALP level. In contrast, none of the treatments significantly affected the rat plasma ALP level (Fig. 4a). The CCl4 treatment alone elevated rat liver MDA level but not significantly compared with the other treatment groups (Fig. 5a). Co-treatment of CCl4 with either ferulic phthalate or maleate decreased (p>0.05) the level of MDA in rat liver. Furthermore, the co-treatment of CCl4 with either ferulic phthalate or maleate reduced rat plasma MDA level compared with CCl4 alone (Fig. 5b). Administration of CCl4 alone did not significantly affect the level of GSH in rat liver compared with the other treatment groups (Fig. 6a). Although co-treatment of CCl4 with either ferulic phthalate or maleate increased the GSH level, the increase was not significant. Furthermore, none of the treatments significantly altered the rat plasma GSH level (Fig. 6b). Administration of CCl4 decreased rat liver SOD level, though not significantly compared with the other treatment groups (Fig. 7a). However, co-treatment of CCl4 with either ferulic phthalate or maleate significantly elevated rat plasma SOD level compared with CCl4 alone (Fig. 7b).
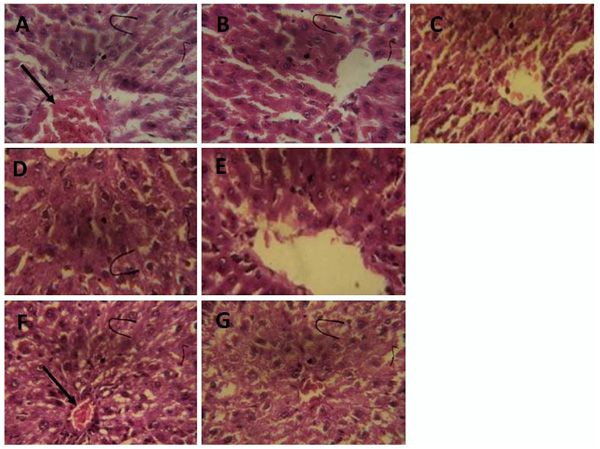
3.3. Histopathological Examination
The histopathological evaluation of rat hepatic sections shows that CCl4 treatment alone caused infiltration of the hepatic nodule (Fig. 8a). However, ferulic acid, ferulic phthalate or ferulic maleate markedly improved the CCl4-induced hepatic distortions (Fig. 8b-g) restoring it to near normal as seen with the group given only distilled water.
4. DISCUSSION
Ferulic Acid (FA) is a phenolic compound that exhibits a wide range of therapeutic effects majorly due to its antioxidant property. There have been recent efforts to maximize the therapeutic value of FA by way of structural modification of FA to form FA derivatives in order to enhance physicochemical, antioxidant and therapeutic prospects. Recent investigations to maximize the therapeutic benefits of ferulic acid have demonstrated that structural modification could enhance physicochemical and antioxidant property of ferulic acid [7, 8, 17]. To prove the principle, we synthesized two new derivatives of ferulic acid and evaluated their protective effect against CCl4-induced liver injury in Wistar rats.
The preparation of ferulic maleate afforded a white powder which melted over a range 140-142 oC with a yield of 72%. In the FT-IR spectrum, the O-H stretching of the two terminal carboxylic acids was observed as a broadband at 3431 cm-1. The characteristic bands of the aromatic and aliphatic C-H stretching appeared in the range of 3010 and 2933 cm-1 respectively. The appearance of the stretching vibration at 1685 cm-1 corresponding to the carbonyl stretching of ester confirms the success of the reaction. This agrees with the previous report [36]. The carbonyl stretching frequency of the ester has thus reduced the electron delocalisation of the conjugating pie bond. The bands which correspond to the C=C stretching, C-H (in-plane bending) and C-O-C stretching were observed at 1431, 1273 and 1209 cm-1 respectively. Other bands were below the fingerprint region. The compound was subjected to mass spectrometry analysis using the electron impact mode. In the mass spectrum, the decarboxylation of the two carboxylic acid followed by reduction afforded the ion peak at m/z 206 calculated for C12H14O3 while further cleavage of the ester gave the fragment C9H10O+ ion depicted at m/z 135. The benzene fragment ion was observed as [C6H6-1]+ at m/z 77.
The synthesized ferulic phthalate with melting point 183-184oC which was obtained as a white powder had Rf 0.76 when developed in dichloromethane solvent system. The characteristic FT-IR spectra exhibited bands at 3437, 3014, 2910, 1693 cm-1 which corresponds to the O-H, aromatic C-H, aliphatic C-H and C=O stretchings respectively. The shifting of the carbonyl stretching to 1693 cm-1 with the corresponding C-O-C stretching at 1207 cm-1 also confirms the success of the synthesis as previously indicated. In the corresponding mass spectrum, the molecular ion peak was observed at m/z [M-1]+ 341 which corresponds to C18H14O7. The reduction of the vinylic carboxylic afforded C18H14O6+ was observed at m/z 327 while further decarboxylation led to the generation of the pear5k at m/z 282 corresponding to C17H14O4+. The further fragmentation of that afforded C9H11O at m/z 135.
Findings show that treatment with CCl4 alone caused mild liver injury as reflected in the alteration of liver function indices. In particular, CCl4 appreciably elevated rat plasma ALT level, a marker enzyme for the evaluation of the integrity of the liver [37, 38]. The significant (p<0.05) increase in the plasma ALT levels of the treatment group administered CCl4 alone may be as a result of a compromise in the liver membrane integrity with a corresponding leakage of the enzyme into the plasma. This finding also agrees with investigations reported by Kodovanti et al. [39], demonstrating the hepato-toxic potential of CCl4. Meanwhile, ferulic acid and its derivative, ferulic phthalate or maleate significantly (p<0.05) ameliorated the increase in plasma level of ALT caused by CCl4 suggesting the potential to protect against liver damage. In addition, CCl4 alone and co-treatment with FA, FP or FM inconsistently altered AST and ALP levels. The reason for this is not known yet. Furthermore, the elevated MDA level caused by CCl4 indicates lipid peroxidation which might be a fallout of oxidative cellular damage. The increase in the level of MDA might be due to the generation of free radicals thereby resulting in the peroxidation of membrane biomolecules. Moreover, lipid peroxidation has been implicated in the development of hepatic injury by the free radical derivatives of CCl4, and this has been linked with cell membrane damage and consequent hepatotoxicity [40]. However, co-treatment of CCl4 with either ferulic acid or ferulic phthalate or maleate did reduce the level of MDA and this reduction was more appreciable in rat plasma. This finding is consistent with an earlier report which demonstrated that phenolic amide exerted antioxidant action by inhibiting lipid peroxidation [41]. In addition, a separate report recently showed that the consumption of polyphenols protected against oxidative damage in rats [42]. Taken together, these findings further support the medicinal prospects of polyphenolic compounds.
Also, CCl4 has been shown to alter the antioxidant profile of the liver including the antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione transferase (GST) [43]. In the present study, CCl4 did not significantly alter the GSH level, however, it is not impossible that adaptive mechanism might be at play if we consider that GSH level in rat liver and plasma were virtually at the same level. The co-treatment of CCl4 with either ferulic phthalate or maleate did elevate GSH level though not significantly, which may indicate the capability of these ferulic derivatives to boost adaptive antioxidant status particularly in the face of the oxidative onslaught. This would agree with the fact that increased intracellular GSH may be a response to oxidative stress, and thus reflect cellular tolerance to oxidizing environments [44, 45]. In such a scenario of an adaptive response to oxidative stress, the GSH synthesis and mobilization may increase in order to dampen acute oxidative onslaught.
SOD is a first line defense antioxidant enzyme which combats O2•−, converting it into oxygen and hydrogen peroxide [46]. The significant (p<0.05) increase in SOD levels seen in the plasma of the treatment groups co-administered CCl4 with ferulic phthalate or maleate might be an outplay of induction mechanism put in place for the generation of SOD, in order to combat the associated reactive species due to CCl4 exposure. SOD is an inducible enzyme, therefore, elevated levels of SOD may partly indicate induction in response to the presence of Reactive Oxygen Species (ROS). This line of opinion would be consistent with a study reported by Adeyemi et al. [47], who linked elevated SOD level to oxidative stress in rats. Meanwhile, co-treatment of CCl4 with either FP or FM might have boosted the induction of SOD in order to counteract the oxidative assault imposed by exposure to CCl4.
The histopathological examination did show that CCl4 exposure alone mildly distorted rat hepatic architecture. In contrast, co-treatment of CCl4 with either ferulic acid or ferulic phthalate improved and restored the rat hepatic architecture. Though co-treatment of CCl4 with ferulic maleate did not completely restore cellular architecture of rat liver, the improvement in biochemical indices suggest that ferulic maleate has the hepatoprotective capability. Ferulic acid is a phenolic compound with antioxidant property and it is reactive towards free radicals such as reactive oxygen species. Our findings agree with previous reports of Rukkumani et al. [48] and Bacanli et al. [49], who demonstrated that ferulic acid protected against oxidative damage in Wistar rats. Conversely, this appears the initial study demonstrating the hepatoprotective effect of ferulic phthalate or maleate in a rat model of liver injury; however, our findings are in consonance with existing investigations which revealed that synthesized phenolic amide offered protection against lipid peroxidation while enhancing hepatic SOD and GSH levels.
CONCLUSION
In the present study, we showed evidence that supports that FA maleate and FA phthalate exhibited strong antioxidant potential as well as protected against drug-induced liver injury in rats. Our findings do not only support the antioxidant as well as the therapeutic potential of these FA derivatives but also to the medicinal value and prospects of phenolic compounds for therapeutic purposes. The findings showed that ferulic phthalate and maleate like their parent compound (ferulic acid), have potent antioxidant property and protected against CCl4-induced liver injury. More so, as an indication of enhanced hepatoprotective effect, the ferulic phthalate and maleate at lower doses (3 and 7 mg/kg bw) compared favourably with ferulic acid (40 mg/kg bw). However, the protection afforded by these ferulic derivatives does not appear to be dose-dependent.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
Authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Authors acknowledge the laboratory staff at the Department of Biochemistry, Landmark University, Omu-Aran, Nigeria for their technical support.


