Vitamin D and COVID-19 Infection
Abstract
Objective:
The COVID-19 epidemic resulted in a global crisis of public health. Therefore, the possibility of prevention, leading to reduced infection and/or an improved disease state, is the subject of intensive attention. The novelty of this study is the direct evaluation of vitamin D levels with the risk of COVID-19 infection.
Background:
Currently, several nutraceuticals, including vitamin D, beta-glucan, and some minerals, are being studied for their role in stimulating immunity. Our study focused on the relationship between levels of vitamin D in immunodeficient patients and the risk of the development of COVID-19.
Method:
In this study, patients were supplemented with vitamin D.
Results:
In a group of 71 patients, we found that patients with vitamin D levels below 30 ng/ml had an increased risk of COVID-19 development and more severe disease progress. In patients with blood levels over 40 ng/ml, we consistently found high levels of protection against COVID-19 infection.
Conclusion:
The most important finding is that vitamin D levels above 40 ng/ml result in the reduction of risks of serious clinical manifestation of COVID-19 infection.
1. INTRODUCTION
Vitamin D (VD) has been the focus of intensive studies worldwide. Most of the new data are associated with the well-established connection between vitamin D deficit and a wide spectrum of diseases. More information has suggested the universal effects of VD and its various roles in immune mechanisms, protein synthesis, muscle, cardiovascular and skeletal functions, cell growth, and inflammatory response [1-11]. The basics of individual mechanisms mentioned above are reaching of optimal level of VD and its regular monitoring. Despite the fact that the last decade brought a huge amount of information on the role of VD in homeostasis, one can point out that this vitamin (or more precisely prohormone), which is involved in the hormone regulating cell growth and functions of the immune system, still requires more attention from both scientists and physicians. Ignoring these findings and underestimating the effects of persisting deficiency of VD result in a significant reduction of immune system regulation [1-3, 12, 13]. Vitamin D plays a role in the regulation and quality of both native and adaptive immunity and significantly improves the regulation of mechanisms of cellular immune responses, particularly via induction and activation of Tregs [3, 5, 10, 14-18].
The COVID-19 pandemic is still causing serious concerns worldwide [13, 19, 20] and the end of this pandemic is still far away [12, 21]. The global crisis of public health is caused by insufficient knowledge of how to protect against COVID-19 infection. Knowing all preventive steps is imperative for the final solution [8, 11, 12, 14, 19-21]. Many authors have agreed that there is a strong need to start the three-level program of prevention, diagnosis, and treatment of this disease [22]. An adequate approach seems to be using nutraceuticals, including minerals, vitamins, and various immunomodulatory agents, all resulting in improved immune responses. The most indispensable are vitamins C and D, calcium, zinc, magnesium, and copper [14, 19, 20, 23-26]. In the past, selenium supplementation has also been recommended. However, the latest observations suggested that this supplementation can be ignored in our study population, as the changes in nutrition in the Czech Republic have improved the levels of this element. The nutrients mentioned above have been used in our patients for a long time [13, 27]. We consider the monitoring of the nutritional habits of our patients to be the bases of healthcare and prevention [20]. Targeted nutritional interventions are considered the backbone of prevention, and their use is one of the most important steps in the reduction of infectious diseases [13, 19, 21].
Research in numerous countries demonstrated the effects of vitamin D supplementation on reducing the risk of viral infections [21], including common viral infections, flu, and COVID-19. Vitamin D plays a role in all phases of infection via improvements of the mucosal barrier and both native and adaptive immunity. Vitamin D also activates dendritic cells, which helps to further regulate immune reactions [5]. In addition, VD changes the binding and adhesion of viral particles and increases nonspecific immune response by induction of antimicrobial peptides, such as cathelicidin and LL-37. Cellular response mechanisms are stimulated by the production of pro-inflammatory cytokines (IL-2 and IFN-gamma) and improved production and regulation of Tregs. Vitamin D supplementation has been found to increase the expression of genes affecting anti-oxidative mechanisms, such as glutathione reductase and glutamate-cysteine ligase. All these steps are involved in the anti-inflammatory effects of VD, which is subsequently recommended for complex treatment of viral infections [1, 3, 4, 8, 11, 13-16, 18, 21, 22, 28-37].
In this study, we evaluated immunodeficient patients from our department. As part of complex treatment, we focused on the quality of nutrition and its relationship with common diseases and also accompanying diseases. The role of VD is supported not only by our findings but also by the findings of several studies conducted in several European countries on VD levels in relation to the lethality of COVID-19 infection (Table 1). Clearly, countries, where VD supplementation was monitored and supported, showed lower COVID-19 mortality.
2. MATERIALS AND METHODS
We evaluated 71 patients in our department with the diagnosis of primary or secondary deficit of immunity. In all patients, we repeatedly monitored the quality of immune functions. Monitoring consisted of a full spectrum of humoral and cellular immune reactions (50 parameters in total) and levels of vitamin D and minerals.
We tried to improve immune reactions by targeted supplementation with a combination of minerals; a list of a combination of minerals was prepared over 30 years ago and precisely defined levels of calcium carbonate, magnesium lactate, zinc lactate, and copper gluconate. In addition, we used VD supplementation with 0.5 mg/ml of vigantol (Lusomedicamenta Sociedade Technica Farmaceutica, Portugal). The doses were determined according to the phototype, nutritional conditions, basic disease/s, sunshine exposure, types of therapy, and other aspects.
| Countries | Mean Vitamin D | Death / 1 M Population | ||
|---|---|---|---|---|
| nmol/L | ng/mL | May 2020 | Dec 2021 | |
| Sweden | 76 | 30 | 380 | 1491 |
| Norway | 68 | 27,2 | 43 | 220 |
| Finland | 68 | 27,2 | 55 | 262 |
| Denmark | 65 | 26 | 97 | 528 |
| Netherlands | 60 | 24 | 336 | 1191 |
| Belgium | 50 | 20 | 790 | 2390 |
| UK | 47 | 18,8 | 526 | 2151 |
| Ireland | 56 | 22,4 | 319 | 1163 |
| Germany | 50 | 20 | 98 | 1294 |
| Switzerland | 45 | 18 | 219 | 1368 |
| France | 60 | 24 | 431 | 1854 |
| Italy | 50 | 20 | 535 | 2247 |
| Spain | 42 | 18,8 | 596 | 1896 |
| Portugal | 39 | 15,6 | 115 | 1847 |
| Czech Republic | 60 | 24 | 28 | 3280 |
| Hungary | 60 | 24 | 48 | 3899 |
| Poland | 45 | 18 | 46 | 2422 |
3. RESULTS
In all initial tests, the levels of vitamin D were reported to be below 20 ng/ml. The levels of VD were regularly monitored at the same time as levels of Ca, Mg, Zn, and Cu. The dose of vitamin D was recalculated after 3 months of the study after additional evaluation of the vitamin D levels. All samples were evaluated immediately after collection as part of the monitoring.
During our observations, we diagnosed 39 patients (54.9%) with COVID-19 infection; the rest were negative (both by PCR test and clinical evaluation). Fig. (1) shows a correlation between COVID-19 positivity and VD levels in our patients. In the group with VD deficiency (3 - 19.9 ng/ml), COVID-19 infection was found to be in 100% of patients; in the group with an insufficient level of VD (20.0 - 29.9 ng/ml), 85.7% of patients were infected. In the group with normal VD levels (30.0 - 39.9 ng/ml), 58.8% of patients were found to be positive, all with only minor clinical manifestations. In the last group of 24 patients with VD levels above 40 ng/ml, we found only two COVID-19 cases, both very mild and without any respiratory problems. In both cases, a one-week hospital stay resulted in complete health. In one of these patients, we found a reduction of 35.8 ng/ml in VD levels, which was easily improved by VD supplementation (Fig. 1). However, it is interesting to note that this patient generally was not in good health: BMI 43.5, diabetes type II, diabetic nephropathy, and vaccination against repeated streptococcal skin infection.
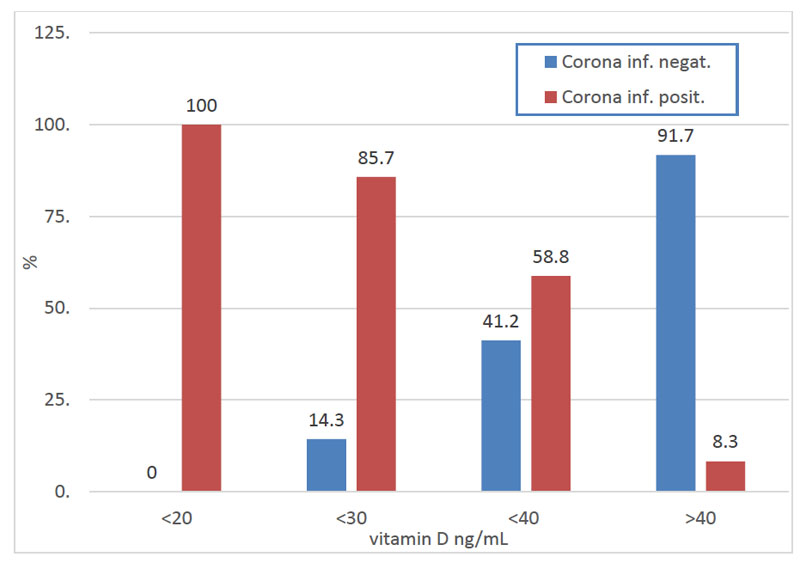
COVID-19: Marked clinical manifestation of diseases (Corona infection positive); no clinical manifestation (Corona infection negative).
4. DISCUSSION
An adequate diet can fully cover all energetic needs, biosynthetic substrates, and regulatory molecules if necessary for the prevention and treatment of infectious diseases. Various vitamins (mostly A, B, C, D, and E) and trace elements (Zn, Cu, Se, Fe, Ca) are crucial for supporting the immune system and reducing the stress caused by different factors. Numerous studies demonstrated a significant reduction in the risk of development of infectious diseases by nutrition [14, 19, 20].
Nutrition in the Czech population is generally poor quality, so we cannot expect an adequate intake of vitamins, micronutrients, amino acids, and fatty acids. Various aspects of the lifestyle (smoking in over 35% of the population), industrial and environmental contaminations (some parts of the Czech Republic are considered to be the “European black spot”) play a role in nutritional deficits. Low levels of VD and calcium in some regions might also be caused by various medications (statins, glucocorticoids, cytostatics, anti-diabetics, diuretics, laxatives, and anti-estrogens) [24], which corresponds to the higher use of these drugs in regions with an increase incidence of many diseases. It is important to note that hypocalcemia increases the risk of serious complications during COVID-19 infection [23]. Therefore, we propose to monitor levels of individual components for at least 6 months and use targeted supplementation. In agreement with the findings of other authors, we conclude that nutritional intervention is a significant factor in improvements of various immune reactions [2, 4, 19, 20, 22, 25]. Table 1 summarizes the unsatisfactory situation regarding insufficient levels of VD in the European population. Only in countries interested in food supplementation and with higher consumption of food with high VD content, this situation seems to be better, which is also reflected in lower COVID-19 mortality. However, it is important to note that these conditions are similar in most countries [2, 4, 5, 17, 21, 34, 27, 38]. These results might be somehow less accurate due to the fact that most of the VD values were obtained during hospitalization and did not reflect age, sex, phototype, and actual or long-term treatment. The situation in the Czech Republic, as shown in Table 1, is based on measurements obtained in two regions and might significantly differ from the situation in Nothern Bohemia, known for heavy pollution, lower living standards, and different structures of the population. In our group of 540 patients, we found 19.8 + 6.2 ng/ml levels of VD; in 51 patients with diabetic retinopathy, we found average VD levels of only 13.75 + 5.25 ng/ml. Although sunshine is responsible for at least 85% of total VD levels, in Northern Bohemia, its effect reduces by pollution, physical stress, and unhealthy lifestyle [7, 9, 10, 24, 29, 31, 38, 39]. The nutritional role of VD levels in the Czech Republic, in general, and in Nothern Bohemia, in particular, is important compared to other European locations. Yearly fish consumption in the Czech Republic is less than 4 kg, and other nutritional components change the VD levels only marginally. Therefore, we propose the importance of nutritional changes, particularly increased VD supplementation, not only as an important preventive treatment [4, 7, 14, 26] but also as adjuvant therapy for numerous diseases [2, 3, 37, 38], as VD is an important part of mechanisms influencing various physiological processes, including immune mechanisms [2, 4, 16]. Another important advantage of VD supplementation is the regulation of intestinal immunity [6]. Concerning this, we propose combining VD supplementation with beta-glucan, as several research groups described its synergetic positive effects on immunomodulation and postinfectious complications [8, 40, 41].
CONCLUSION
The present study confirmed findings by other groups demonstrating the positive role of supplementation with VD. Our findings demonstrated that the optimal values of vitamin D must be over 40 ng/ml. Although it cannot be stated that VD will cure all health problems, its positive effects on the overall health of the population cannot be overlooked. Furthermore, besides VD supplementation, we hypothesize that CD supplementation for children and the elderly will have significant positive effects.
LIST OF ABBREVIATIONS
| VD | = Vitamin D |
| BMI | = Body Mass Index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
Vetvicka V is on the editorial advisory board of the journal The Open Biochemistry Journal.
ACKNOWLEDGEMENTS
Declared none.


