All published articles of this journal are available on ScienceDirect.
Activity of Antimicrobial Peptide; Cathelicidin, on Bacterial Infection
Abstract
Antimicrobial peptide is an effector molecule from the natural immune system which plays a central role in defense as an antimicrobial. Cathelicidin is one of the antimicrobial peptides. Human only has one cathelicidin antimicrobial peptide called LL-37 or hCAP18. The detailed mechanism on CAMP (Cathelicidin Antimicrobial Peptide) gene regulation is still unknown, however, cathelicidin is found to have upregulation when there is bacterial infection. The most effective expression inducer of CAMP gene is 1,25-dihydroxyvitamin D3 (1,25(OH)2 D3), which is the active form of vitamin D. Vitamin D mediates cathelicidin synthesis through the expression of Vitamin D Receptor (VDR), then the interaction activates CAMP gene to express cathelicidin. The work mechanisms of cathelicidin against bacterial infection include damaging the bacterial cell membrane, inducing autophagy process of macrophage cell, neutralizing LPS produced by bacteria, and chemotactic activities of PMNs, monocytes and lymphocytes.
1. INTRODUCTION
Quick and effective response to pathogen infection is very important for the survival of all organisms. Defense mecha- nism which has been evolved to meet this need is the production of various microbial peptides. There have been over 100 microbial peptides that have been isolated from animals and plants [1, 2]. These peptides play major roles in the immunity of invertebrates, while in vertebrates, these peptides act as the first line of defense against the invasions of patho- gens and in controlling natural floras [2, 3]. The importance of these peptides can be concluded from their specific localization in sites invaded by microbes and locations where phagocyte cells work. Antimicrobial peptides are produced in the epithelial cells of amphibians [3], mammals [4-6] and insects [2, 7], secreted into internal body fluid in arthropods [2, 7] and stored in cytoplasmic granules of phagocyte cells in mammals and birds [8-11].
2. ANTIMICROBIAL PEPTIDE
Antimicrobial peptide (AMP) is a molecular group pro- duced by cells and tissues in the body of living organism, which has an important role as the defense system of the body. Prokaryotes and human are known to produce AMPs in their bodies [1]. AMPs are known to actively act as antimicrobial and antifungal. Some of them even have antiviral and anti-parasite effects [2].
AMPs originate from a bigger precursor and consist of a chain of 100 amino acid, which is cationic at neutral pH, which is amphiphatic (has both hydrophilic and hydrophobic properties in the structure), and has a broad-spectrum of antimicrobial activity. High antimicrobial activity and relatively low cytotoxicity are due to the basic difference in the membrane lipid composition of bacterial and multicellular organisms. Outside the bacterial membrane, there are many negative phospholipids, while outside the multicellular organism membrane, there is no charge. Therefore, the cationic character of AMPs defines its specificity to bacterial membrane [3] (Fig.1).
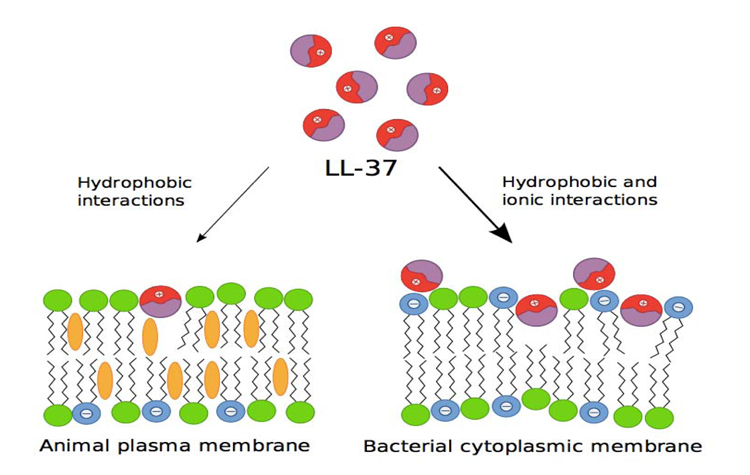
Moreover, AMPs vary greatly in terms of structure. For example, an AMP consists of a single α-helix peptide chain, β-sheet bond and disulfide which contains peptide. In humans, there are two main classes of AMPs, i.e. defensins and cathelicidin. Several different defensins have been found in humans, but there is only one type of cathelicidin [3].
Defensin is an AMP family with a characteristic of having β-sheet bond and three disulfide bridges framework. Defensin has widespread antimicrobial activities and is abundantly expressed by human epithelial cells and blood cells. There are two defensin families which are expressed in human, i.e. α and β-defensin, which are categorized by cysteine location and disulfide-bridge pattern. The most prominent α-defensin is expressed by Paneth cells in the small intestine and neutrophil, while β-defensin is more expressed by various blood and epithelial cells. Some AMPs in human which have been identified are presented in Table 1 [3].
3. CATHELICIDIN ANTIMICROBIAL PEPTIDE (CAMP)
AMP is an effector molecule from the natural immune sys- tem which plays a central role in defense as an antimicrobial. Cathelicidin is a type of antimicrobial peptide [7]. It is a small molecular peptide (consisting of 12-100 amino acid) which has broad-spectrum of antimicrobial activity and is estimated to have a function in the natural immune system as the first defense against microorganisms [8].
Cathelicidin has N-terminal prosequence, followed by C-terminal variable sequence, which has high microbial activity when formed enzymatically. The name cathelicidin is used for this antimicrobial peptide group because the prosequence’s structure is very identical with a protein called cathelin [9]. Cathelicidin, in varying amounts, has been found in every mammal species to date.
Humans and rats have one cathelicidin, while cows and pigs have several cathelicidins [10]. Human and rat cathe- licidins are encoded by similar genes and α-helix struc- ture, antimicrobial spectrum, and distribution in similar tissues, so rat cathelicidin is often used as in vivo analysis of human cathelicidin [11]. Overview of the Cathelicidin antimicrobial peptides of various species are presented in Table 2.
As shown in Table 2, human only can have one cathelicidin antimicrobial peptide called LL-37 [12] or hCAP18, which is pro-peptide [13]. The name LL-37 is based on mature peptide, consisting of two dua leusin residuals and containing a total of 37 amino acids. Meanwhile, hCAP-18, originates from CAP18 rabbit homologous, an 18 kDa cationic antimicrobial peptide. Mature peptide will be referred to as LL-37, precursor LL-37 as pro-LL-37, precursor with peptide signal as pra-pro-LL-37 and the gene as CAMP.
Cathelicidin in human is called human Cathelicidin Anti-microbial Protein (hCAP) which consists of an active C-terminal domain and 37 amino acids, so it is also referred to as LL-37 which reflects the length of the residual of 37 amino acids with residual of 2 leusins [7, 13]. Human cathelicidin anti-microbial protein has a molecular mass of 18 kDa, hence it is also referred to as hCAP18 [13, 14]. An in vitro study has confirmed the anti-infectious role of hCAP18 and its close relation with vitamin D metabolism and other mechanisms of the natural immunity [7].
4. MOLECULAR STRUCTURE OF CATHELICIDIN ANTIMICROBIAL PEPTIDE
Human cathelicidin anti-microbial protein has a molecular mass of 18 kDa, so it is also referred to as hCAP18 [13, 14] with α-helical structure and 2 disulfide bridges between 2 cysteine molecules (C85-C96 and C107-C124) [14]. hCAP18 molecule contains inactive N-terminal domain (the location of cathelin) and C-terminal AMP (LL-37) [15]. Cathelin is the inactive domain of cathelicidin, activated by the work of serine protease to bring out a very specific multifunctional domain [7]. Cathelin is a protein from porcine neutrophils which inhibits cathepsin L protease (hence called cath-e-L-in) [16].
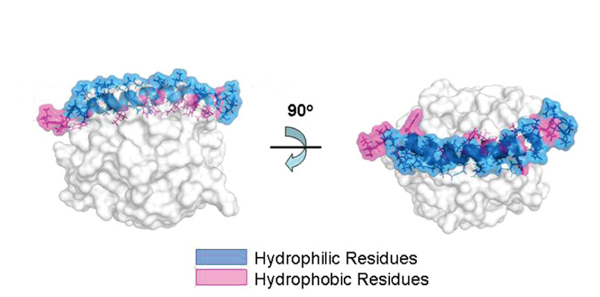
After the activation of hCAP18, LL-37 is released to the plasma, then antimicrobial activity is performed by α-helical structure. Ionic composition, pH or salinity in its environment affect α-helical structure, disturbing the antibacterial activities of LL-37. In higher pH plasma, α-helical structure will be maintained and guarantee microbial peptide activity. With pH<7.2, most peptides are open and inactive. Human cathelicidin antimicrobial protein has a amphiphilic structure with hydrophobic and hydrophilic fragments enabling the interaction in aqueous environment and lipid membrane [15].
LL-37 adopts the α-amphipathic helix structure in solution which contains physiological salt concentration [17]. Because of the positive charge of the membrane, it has a strong affinity to the lipid membrane of bacteria which has negative phospholipid outside the membrane. Various studies show that LL-37 damages bacterial membrane through carpet-like mechanism [6, 18] and doesn’t form pores as performed by complements and perforin (Fig.2) [19].
| Species | Cathelicidin Antimicrobial Peptide |
|---|---|
| Horse | eCATH-1, -2, and -3 |
| Pig | Prophenin, PR-39, AMPP-23, -36, & -37, Protegrins 1-5 |
| Cow | Indolicidin, dodecapep, Bac5 & 7, BAMP-27, -28 & -34 |
| Lamb | s-dodecapep, oaBac6 & 11, sBac5 & 7.5, SAMP-29 & -34 |
| Goat | chBac5 & 7.5, chMAP-28 & -34 |
| Money | RL-37 |
| Human | LL-37 or hCAP18 |
| Rabbit | CAP18 |
| Mouse | CRAMP |
| Rat | rCRAMP |
5. GENETIC STRUCTURE OF CATHELICIDIN ANTIMICROBIAL PEPTIDE OR CATHELICIDIN ANTIMICROBIAL PEPTIDE (CAMP) GENE
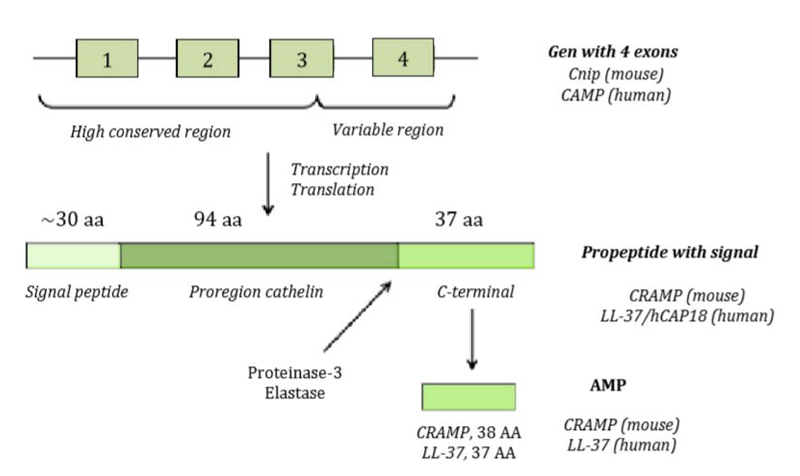
The genetic structure of hCAP18 or CAMP gene with the length of 2 kb consists of 4 exons and 3 introns as shown in Fig. (3) [9]. Exons 1-3 encode peptide signal and cathelin domain, while the 4th exon shows the location which is split and releases LL-37 active peptide [9, 20]. Protolithic breaks inactive precursor molecule to release mature AMP C-terminal from cathelin prodomain done by elastase or proteinase-3 during degranulation of activated neutrophils (Fig. 3).
Cathelicidin Anti-Microbial Peptide (CAMP) gene is located in chromosome 3 (3p21) near other genes which have the same functions, e.g. gene for macrophage colony stimulating factor 1 (an antiviral cytokine), gene for hepatocyte

growth factor like protein (a multifunctional factor required in repairing tissue and angiogenesis), gene for collagen VII alpha-1 polypeptide (a protein responsible for tissue integrity), and genes for natural killer-tumor recognition (a molecule involved in T-killer lymphocyte activity). According to a study, cathelicidin up-regulates 49 genes, control expressions of some chemokines or chemokine receptors (CXCR-4, CCR2) and some cytokines (IL-8) [20]. The transcription of CAMP gene is increased during bacterial, viral, fungal or protozoa infection. Calcitrol [1,25(OH)2D] is a very strong inducer of cathelicidin mRNA transcription [21].
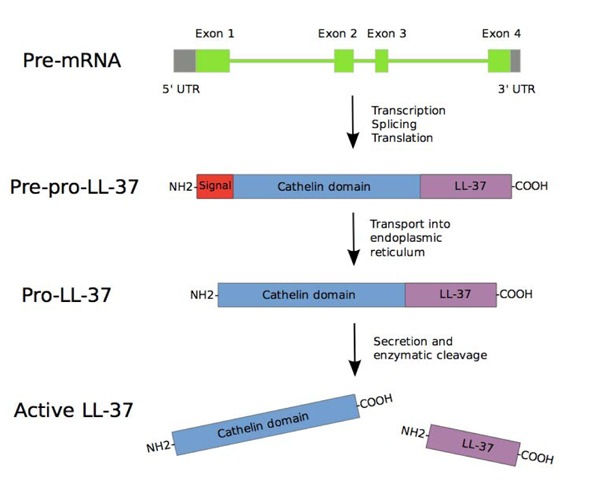
CAMP gene consists of four exons. The first three exons encode signal peptide and pro cathelin section, while the fourth exon encodes a processing site and peptide. After the transcription and translation process, pre-pro-LL-37 is delivered to endoplasmic reticulum, while signal peptide is split during the process. Depending on the type and condition of the cell, pro-LL-37 is then transported further and stored in granules or is immediately secreted. The secreted Pro-LL-37 is then transformed into LL-37 by proteinase 3 enzyme produced azurophil neutrophilic granules [22] (Fig. 4).
Cathelicidin antimicrobial peptide is synthesized by several cells, e.g. polymorphonuclear (PMN) cells, monocytes, lymphocytes, mastocytes, and epithelial cells, such as keratinocyte or cells of gastrointestinal tract, respiratory tract, and genital endothelium. In a healthy person, hCAP18 plasma content is 50-80ng/mL. Cathelicidin is synthesized and stored as a preproprotein. With antigen stimulus present, the precursor enzymatically split terminal peptide LL-37 which is a biologically active domain, then LL-37 is released to plasma, extracellular space or saliva, milk, sudoriferous secretion, seminal fluid, and amnion. Human cathelicidin antimicrobial protein 18 has an important role in fast natural defense through synthesis in sentinel cells, e.g. epithelial and endothelial cells, or quick release into plasma by blood cells after specific stimulation.
Vitamin D mediates the synthesis of cathelicidin through the expression of Vitamin D Receptor (VDR). Microbial molecules, such as lipoteikoic acid, peptidoglycan, and atypical lipopolysaccharide from Leptospira and Porphyromona gingivalis specie, lipomanan from mycobacterial family, a number of viral and fungal antigens, activate toll-like receptors 2 (TLR-2), then the receptor will trigger a response mediated by vitamin D in the form of interaction between 1,25(OH)2D and VDR. This interaction will activate the CAMP gene to express cathelicidin. The increase or reduction of VDR expression will change cathelicidin level. In the presence of stimulus by infection, vitamin D level also increases the TLR-2 expression, enabling increased response to TLR activation. Some gene polymorphisms which encode TLR-2 or VDR play a role in serious sepsis caused by Gram positive bacterium or infection by mycobacterium. This evidence sets vitamin D as the most important factor in cathelicidin regulation and may explain the antimicrobial work connected with vitamin D [23]. Schematically, cathelicidin synthesis is shown in Fig. (5).
6. REGULATION OF CAMP GENE EXPRESSION
The detailed mechanism on CAMP (Cathelicidin Antimicrobial Peptide) gene regulation is still unknown, however, cathelicidin is found to have upregulation when there is bacterial infection [24]. It is shown that products of bacteria will increase Cathelicidin expression in cultured human cells [25], indicating the role of Cathelicidin in fighting infection. Further evidence shows the role of Cathelicidin in the defense of bacteria, i.e Shigella [26] and Neisseria gonorrhoeae [27] cause downregulation of Cathelicidin as a part of the mechanism of the bacterial invasion.
The most effective inducer of CAMP gene expression is 1,25-dihydroxyvitamin D3 (1,25(OH)2 D3), which is an active form of vitamin D. 1,25(OH)2 D3 works through vitamin D Receptor (VDR) which then binds vitamin D responsive element (VDRE), around 500 bp of CAMP gene upstream promoter [23, 28]. Furthermore, histone deacetylase inhibitor butirate and trichostatin A are proven to induce CAMP gene expression [23, 29]. A recent study [30] shows that the substance only works directly on CAMP promoter by increasing histone acetylation and therefore opening chromatin structure for transcription. All identified substances which can increase CAMP gene expression are summarized in Table 3 [23,31,32] .
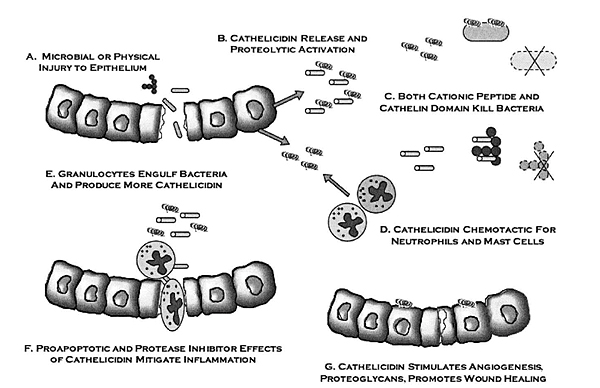
7. CATHELICIDIN WORK MECHANISM
Cathelicidin anti-microbial peptide has multiple works. Some studies report that the works of cathelicidin are: antimicrobial activity [33], PMN chemotactic, monocytes, lymphocytes, and mastocytes activities [34], histamine release by mast cell [35], gene expression stimulation [20], antitoxic activity related with lipopolysaccharide which is Gram negative bacillus endotoxin [20], angiogenesis [36], activation of epithelial cells during trauma and reepithelization of skin during skin infection [37], and regulation of dendritic cell differentiation [38] (Fig. 6).
8. CATHELICIDIN ANTIMICROBIAL MECHANISM
Most antimicrobial effects directly from cathelicidin can be related to its physical structure and cationic and hydrophobic properties [39]. N-terminal helix plays a role in chemotaxis and defense against proteolisis, while C-terminal helix is responsible for antimicrobial effect. Cathelicidin goes toward microbial membrane, then covers the surface for the microbial membrane, and perforates it, forming pores on the membrane which eventually destroys bacterium. Cathelicidin basically binds cell membrane which contains lipopolysaccharide (Gram-negative) or teichoic acid (Gram-positive) with negative charge, unlike zwitterionic eukariotic membrane. Cathelicidin also has antiviral capability with interaction between capsule membrane and protein capsid.
Cathelicidin approaches the bacterial membrane in oligomeric forms, which affects the subsequent interaction and mode of permeabilization. This has a marked effect on the antimicrobial activity, because the monomeric peptide is less subject to sequestration by serum or medium components, or components of the bacterial outer cell wall [40]. Cathelicidin can bond with lipoteikoic acid and lipoarabinomannan, prevent macrophage activation in staphylococcus infection and tuberculosis [20]. In certain cases, some protolithic enzymes from resistant bacteria can damage cathelicidin and other antimicrobial peptides [41].
The induction of autophagy process in human monocytes or macrophages is a mechanism which takes into account the ability of Cathelicidin to kill intracellular pathogens. Autophagy is a very primitive biological process which serves to ensure cell cytoplasmic homeostasis [42]. During active autophagy process, some cytoplasmic materials are trapped inside double membrane vacuole called autophagosome, then join lysosome, into autophagolysosome. In it, lysosome enzyme destroys all trapped materials. Autophagy initially serves to recycle nutrition from damaged molecules, but now it is known that autophagy is involved in various processes, including microbial activities [43, 44].
Cathelicidin is an important protein in forming and running the functions of autophagosomes and autophagolysosomes in human monocytes [42-44], but not the only one, because there are many other mechanisms which induce autophagy released from calcitriol-cathelicidin system which has been found. Cathelicidin induces the expression of two key factors for the development of autophagy, beclin-1 and autophagin (Atg)-5 [42-45]. Both proteins, beclin-1 and autophagin (Atg)-5, are important for the maturation process of autophagy and combination of autophagosom and lisosome. Autophagy mediated by curcumin, cell fasting, or raAMPycin is not regulated by calcitriol-cathelicidin system [45].
Cathelicidin has additional effects which may vary depending on local concentration [45]. It has been proven in vitro, for example, that at a dosage less than 1 mol, Cathelicidin helps inducing neutrophil chemotaxis and survival of neutrophil, and stimulating angiogenesis and fibroblast migration and proliferation (useful effects in healing wounds), while very high concentration, usually outside of the one achieved in normal response, has cytotoxic and pro-inflammatory effects [45].
Beside bactericidal effect by damaging bacterial membrane, hCAP18 also can neutralize LPS produced by Gram negative bacteria, protection against endotoxic shock through three mechanisms [46]: [a] inhibiting TLR-4 dendritic cell receptors, thus preventing its activation and response to LPS, [b] blocking the release of TNF-α at CD14 lymphocyte level, and [c] blocking other compounds in inflammatory process, e.g. nitric oxide, tissue factor, PGE2, chemokine, etc. Other study also reports that cathelicidin has antibiofilm effects against community-associate and hospital isolated methicillin - resistant Staphylococcus aureus (MRSA) strains [47].
As an antimicrobial against viruses, bacteria, and fungi, the performance of cathelicidin is fast and not selective. Cathelicidin shows different effects at different levels. For Gram positive bacteria, the antibacterial effect is seen at 0.75µM, while for Gram negative at >5µM, and for chemotactic activity at 10µM. >15µM level is cytotoxic and should be quickly deactivated by binding with plasma lipoprotein (apolypoprotein A). The antibacterial effect of hCAP18 is related to physical and chemical environments, and also increased by immunomodulator and activities related to chemotaxis [48].
A study on cathelicidin in adults by Gombart et al. [49] reports that a low level of cathelicidin in the circulation is related to higher mortality rate due to infections in patients undergoing hemodialysis [RO=3.7 (IK95% 1.2 to 11.2)]. Another study with cohort design on adults with pneumonia, with convenient sampling, which is performed on winter, reports that both cathelicidin and β-defensin-2 can’t predict mortality. Conversely, vitamin D at 25(OH)D <30 nmol/L increases the mortality risk than 25(OH)D >50 nmol/L with RO=12.7 (IK95% 2.2 to 73.3) [50].
CONCLUSION
Antimicrobial peptides (AMP) are a group of molecules produced by cells and tissues in the body of living beings which play an important role as the body defense system against pathogenic infections. Cathelicidin is a type of antimicrobial peptide which in human is also referred to as hCAP18 or LL-37. Cathelicidin is synthesized by a number of cells, e.g. polymorphonuclear (PMN) cells, monocytes, lymphocytes, mastocytes, and epithelial cells, e.g. keratinocytes or cells of gastrointestinal tract, respiratory tract, and genital endothelium. The detailed mechanism on CAMP (Cathelicidin Antimicrobial Peptide) gene regulation is still unknown, however, cathelicidin is found to have upregulation when there is bacterial infection. The most effective expression inducer of CAMP gene is 1,25-dihydroxyvitamin D3 (1,25(OH)2 D3), which is the active form of vitamin D. 1,25(OH)2 D3 works through vitamin D receptor (VDR) which then binds vitamin D responsive element (VDRE). The work mechanisms of cathelicidin against bacterial infections include damaging bacterial cell membrane, inducing autophagy process of macrophages, neutralizing LPS produced bacteria, and chemotactic activities of PMN, monocytes and lymphocytes.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Authors deeply indebted to supervisor, Prof. Dr. Mochammad Hatta, senior lecturer from the Department of Immunology and Microbiology in the University of Hasanuddin, for a warm support, inspiration and thoughtful guidance in writing this article. Authors acknowledge the University of Muhammadiyah for supporting this publication.


