All published articles of this journal are available on ScienceDirect.
Effect of Unripe Plantain (Musa paradisiaca) and Ginger (Zingiber officinale) on Blood Glucose, Body Weight and Feed Intake of Streptozotocin-induced Diabetic Rats
Abstract
Objective:
To determine the effect of unripe plantain (Musa paradisiaca) and ginger (Zingiber officinale) on blood glucose (BG), feed intake (FI) and weight of streptozotocin (STZ) induced diabetic rats.
Methods:
Twenty four male albino rats were used and were divided into 4 groups of 6 rats each. Group 1 (non-diabetic) and Group 2 (diabetic) received standard rat feed; Group 3 received unripe plantain incorporated feed (810 /kg body weight) and Group 4 received unripe plantain+ginger incorporated feed (710:100 g/kg body weight). The weights and FI of the rats were measured daily throughout the experimentation.
Results:
Groups 3 and 4 rats had 159.52% and 71.83% decreases in BG but 24.91% and 35.32% decreases in weights compared with groups 1 and 2 rats that had 2.09% and 22.94% increases in BG with 13.42% increase and 45.36% decrease in weights respectively. The FI of the experimental rats did not differ significantly from each other (P>0.05) at the end of experimentation. The standard rat feed contained higher amounts of Ca but lower amounts of Mg and Fe compared with the unripe plantain and unripe plantain+ginger incorporated feeds.
Conclusion:
Combination of unripe plantain and ginger at the dose used in the management of diabetes was not very effective compared with unripe plantain alone.
INTRODUCTION
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia, resulting from partial or total destruction of the pancreatic β-cells in insulin synthesis as in type one or peripheral resistance to insulin action which may be due to molecular defects in signal transduction [1], desensitization of insulin receptors for insulin [2] and defects in glucose transporters [3] as in type 2.
Current strategies for the management of the disease include diet, exercise, various oral anti-diabetic drugs and insulin therapy [4].
Although insulin and other synthetic drugs such as biguanides, sulphonylureas, α -glucosidase inhibitors are used for the management of diabetes, these drugs are either quite expensive or associated with unwanted side effects like hypoglycaemia, frequent diarrhoea, hypertension, hepato-toxicity and dyslipidemia [4].
This has led to the increased interest in recent times in the research of plants with anti-diabetic potentials especiallyas plant sources are usually non-toxic with fewer side effects than synthetic sources [5].
Plantain (Musa paradisiaca) is a staple crop in the humid and sub-humid parts of Africa, Asia, Central and South America that is usually eaten as an energy yielding food. Its hypoglycemic actions in diabetic animals have been reported [6, 7].
Ginger (Zingiber officinale) is cultivated in the tropics for its edible rhizome. In a typical Nigerian diet, ginger is one of the most common spices that is used to add flavour to meals [8]. Studies have also shown its hypoglycemic properties [9, 10].
Although unripe plantain and ginger are used individually to manage diabetes mellitus in Nigeria, the possibility of combining them in a typical diabetic diet and the glycemic response elicited as a result of such combination has never been reported.
This therefore led to the study which was aimed at investigating the effect of a dietary combination of unripe plantain and ginger on blood glucose, feed intake and body weights of streptozotocin-induced diabetic rats.
MATERIALS AND METHODS
Plant Materials
Fresh samples of unripe plantain were bought from Umuahia main market in 2013. They were identified by Mr Ibe of the Forestry Department, Michael Okpara University of Agriculture, Umudike (MOUAU). The ginger samples (accession-UGII or black ginger) were obtained at harvest from National Root Crops Research Institute, Umudike, Nigeria that has a National mandate on Ginger. They were also identified by Mr Ibe and by Dr C.O. Amadi, the Co-ordinator, Ginger Program, National Root Crops Research Institute, Umudike. The plants were deposited in the herbarium of MOUAU for authentication.
Processing of the Plant Materials
The samples were properly peeled, soaked in water for about 10 min, washed and oven dried at 70°C to constant weight and processed to flour. The processed flours were pelletized, oven dried at 80°C to constant weight before they were fed to the rats.
The composition of the unripe plantain incorporated feed was: 81% unripe plantain flour, 9% soybean flour, 4% vitamin mixture, 2% salt and 4% groundnut oil while the composition of the unripe plantain + ginger incorporated feed was: 71% unripe plantain flour, 10% ginger flour, 9% soybean flour, 4% vitamin mixture, 2% salt and 4% groundnut oil [11].
The composition of the basal diet given to the non-diabetic and diabetic control rats was comprised of: 19% protein, 10% fat, 31% carbohydrate, 10% crude fiber and 1% calcium.
Chemicals
Streptozotocin (Sigma No.S0130) used was a product of Sigma-Aldrich Chemical Company, UK. Cholesterol and HDL kits used were products of Randox. Every other chemical that was used for the experiment was bought from HosLab, Umuahia, Abia State, Nigeria and was of analytical grade.
ANIMAL EXPERIMENTS
Selection of Animals
Thirty male albino rats of the wistar strain (130.68 to 232.91g) obtained from the animal house of the Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria, were used for the study. The animals were kept in metabolic cages in the animal house of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike, Nigeria. The animals were acclimatized for two weeks to their diets prior to the commencement of the experiment and were maintained under a constant 12-h light and dark cycle and a room temperature of 27-30°C. All animal protocols were approved by the ethical committee of Michael Okpara University of Agriculture, Umudike, Nigeria which was in line with the National Institutes of Health’s Principles of Laboratory Animal Care [12].
Induction of Diabetes
Freshly prepared solution of streptozotocin (0.1g dissolved in 5 mL of freshly prepared sodium citrate buffer 0.1 M, pH 4.5) was injected intraperitoneally to 24 of the rats at a dosage of 65 mg/kg body weight at fasting state while 6 of the remaining rats served as non-diabetic control group. Blood was collected from the tail vein and the blood glucose concentration was analyzed in the STZ treated rats prior to the commencement of the dietary feeding using a blood glucose meter (Double G glucometer, USA) and subsequently, twice in a week, throughout the duration of the experiment. The STZ-treated rats with fasting blood glucose levels > 200 mg/dL after twelve days of induction of STZ were considered to be diabetic and were used for the study.
Experimental Procedure.
The STZ treated rats with stable diabetic condition were then divided into 3 subgroups (groups 2 to 4) comprising of six animals per group while the non-diabetic group formed the first group as follows:
Group 1. Normal rats fed standard rat pellets (Non-diabetic control).
Group 2. Diabetic control rats which also received standard pellets
Group 3. Diabetic rats fed unripe plantain incorporated feeds (810 g/kg)
Group 4. Diabetic rats fed unripe plantain + ginger incorporated feeds (710: 100 g/kg)
The composition of the unripe plantain incorporated feed was: 81% unripe plantain flour, 9% soybean flour, 4% vitamin mixture, 2% salt and 4% groundnut oil while the composition of the unripe plantain + ginger incorporated feed was: 71% unripe plantain flour, 10% ginger flour, 9% soybean flour, 4% vitamin mixture, 2% salt and 4% groundnut oil.
Their diets and water were both given ad libitum for 28days, after which the rats were stunned by blow and sacrificed. The body weights and feed intakes of the rats were recorded on a daily basis using an electronic weighing balance (Model Scout Pro, Ohaus Corporation, USA).
The percentage change in fasting blood glucose (FBG) was calculated as:
The percentage change in weight was calculated as:
PLANT ANALYSIS
The Atomic Absorption Spectrophotometer (Analyst 200, Perkin Elmer, Waltham, MA, USA) was used to analyze the magnesium (Mg), iron (Fe) and calcium (Ca) contents of the flour of the test and standard rat feeds while the potassium (K) contents of the flour were determined using a flame photometer. The crude protein, carbohydrates and fat contents of the flours were determined using the methods of AOAC [13].
STATISTICAL ANALYSIS
Data generated was subjected to analysis using the Statistical Package for Social Sciences (SPSS), version 17.0. Results were presented as the means ± standard deviations of triplicate experiments. One way analysis of variance (ANOVA) was used for comparison of the means. Differences between means were considered to be significant at P<0.05 using the New Duncan Multiple Range Test.
RESULTS
As shown in Table 1, the diabetic control rats recorded 22.94% increase in fasting blood glucose compared to the non-diabetic control rats that had 2.05% increase in fasting blood glucose. Feeding of unripe plantain incorporated feed or a combination of unripe plantain and ginger incorporated feed resulted in 159.52% and 71.83% decreases in blood glucose respectively.
Fasting blood glucose of rats (mg/dL)
| Groups | Animals | Initial | Final | Percentage change |
|---|---|---|---|---|
| Group 1 | Non-diabetic control | 83.75 ± 4.98a | 85.50 ± 12.39a | 2.05 (i/c) |
| Group 2 | Diabetic control | 241.00 ± 69.24b | 312.75 ± 62.56c | 22.94 (i/c) |
| Group 3 | Diabetic + unripe plantain | 252.25 ± 40.32b | 97.20 ± 11.10a | -159.52 (d/c) |
| Group 4 | Diabetic + unripe plantain + ginger | 366.00 ± 24.04c | 213.00 ± 54.56b | -71.83 (d/c) |
Values are means ± SD. a-cMeans with different superscripts along the column are significantly different (P<0.05). i/c - increase ; d/c - decrease
Mineral composition of test and standard rat feeds (%)
| Plants | Mg | Fe | Ca | K |
|---|---|---|---|---|
| Plantain | 0.15 ± 0.03c | 0.41 ± 0.04c | 0.51 ± 0.02b | 0.44 ± 0.02b |
| Plantain+Ginger | 0.10 ± 0.01b | 0.28 ± 0.01b | 0.44 ± 0.02a | 0.52 ± 0.02c |
| Rat feeds | 0.06 ± 0.02a | 0.15 ± 0.04a | 1.04 ± 0.05c | 0.27 ± 0.03a |
Values are means±SD. a-cMeans with different superscripts along each column are significantly different (P<0.05).
Proximate composition of test and standard rat feeds (%)
| Plants | Crude protein | Carbodydrates | Crude fat |
|---|---|---|---|
| Plantain | 14.33 ± 0.50a | 55.96 ± 0.05c | 12.59 ± 0.16b |
| Plantain+Ginger | 16.28 ± 0.03b | 43.12 ± 0.10b | 20.44 ± 0.07c |
| Rat feeds | 15.05 ± 1.15a | 33.00 ± 3.46a | 7.00 ± 1.15a |
Values are means±SD. a-cMeans with different superscripts along each column are significantly different (P<0.05).
The diabetic rats fed unripe plantain incorporated feed had 24.91% loss of weight, the diabetic rats fed unripe plantain and ginger incorporated feeds had 35.32% loss of weight, the diabetic control rats recorded 45.36% loss of weight compared to the non-diabetic control rats that had 13.42% gain in weight (Fig 1).
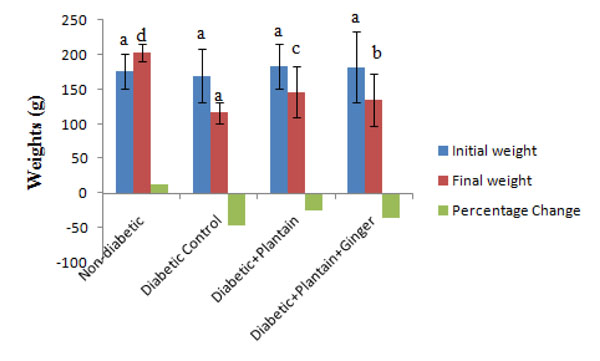
Body weight and percentage change in weight of rats. Values are means ± SD. a-d Means with different superscripts are significantly different (P< 0.05).
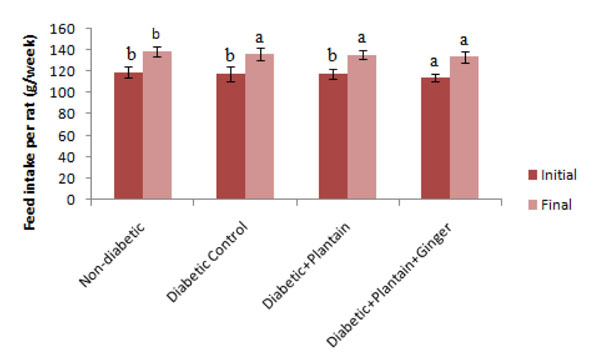
Feed intake per rat.
The initial feed intake of the non-diabetic, diabetic, diabetic rats fed unripe plantain incorporated feed and diabetic rats fed unripe plantain+ginger incorporated feed were 119.40 ± 5.40, 118.00 ± 6.70, 118.00 ± 4.66 and 114.22 ± 3.77 respectively, while the final feed intake of the non-diabetic, diabetic, diabetic rats fed unripe plantain incorporated feed and diabetic rats fed unripe plantain+ginger incorporated feed were 139.20 ± 4.55, 136.40 ± 5.33, 135.66 ± 3.77 and 134.00 ± 5.25, respectively (Fig. 2). There were no significant differences (P>0.05) in the final feed intake of the diabetic control rats compared with the feed intake of the diabetic rats given the test feeds but significant decreases of the feed intake of the diabetic rats compared with the non-diabetic rats (Fig. 2).
Mineral analysis of the test and standard rat feeds showed that the unripe plantain incorporated feed contained significantly higher (P<0.05) quantities of Ca compared with the unripe plantain+ginger incorporated feeds, and significantly higher (P<0.05) quantities of Mg and Fe compared with the unripe plantain+ginger and the standard rat feeds while the standard rat feeds contained significantly higher (P<0.05) quantities of Ca than the unripe plantain and unripe plantain+ginger incorporated feeds while the unripe plantain +ginger incorporated feeds contained significantly higher (P<0.05) quantities of K compared with the unripe plantain or unripe plantain+ginger incorporated feeds (Table 2).
Proximate analysis of the test and standard rat feeds showed that the unripe plantain+ginger incorporated feed contained the highest crude protein contents amongst the samples investigated while unripe plantain incorporated feed had the least though not significantly different (P>0.05) from that of the standard rat feed (Table 3).
The unripe plantain+ginger incorporated feed contained the highest crude fat contents amongst the samples investigated while the standard rat feed had the least (Table 3).
The unripe plantain incorporated feed contained the highest carbohydrate contents amongst the samples investigated while the standard rat feed had the least (Table 3).
DISCUSSION
The choice of 10% ginger being incorporated into the test feeds of the rats of group 4 was because ginger is merely used as a food adjunct or spice (not more than 10% of it is added into foods).
The significant decrease in the fasting blood glucose levels of the diabetic rats fed unripe plantain incorporated feed or a combination of unripe plantain and ginger incorporated feed suggests the anti-hyperglycemic actions of the test plants. However, unripe plantain was found to be more effective in lowering the elevated blood glucose of the diabetic rats compared with its combination with ginger at the dosage used in this study.
The reduction of the body weights of the diabetic control rats despite their increased feed intake is attributed to their hyperglycemic condition, leading to catabolism of structural proteins [14, 15] while the decrease in weight loss after feeding of unripe plantain incorporated feed or a combination of unripe plantain and ginger incorporated feed could be attributed to the ability of the plants to reduce hyperglycemia [14].
Magnesium is a cofactor of the glycolytic enzyme hexokinase and pyruvate kinase. It also modulates glucose transport [16].
Iron influences glucose metabolism, insulin action and it also interferes with insulin inhibition of glucose production by the liver [17].
Calcium is an important component of intracellular processes that occur within insulin responsive tissues like muscle and adipose tissues [18]. Although the standard rat feeds contained higher quantities of Ca than the unripe plantain and unripe plantain incorporated feeds, consumption of this feed by the diabetic control rats did not modulate their elevated blood glucose levels.
Low dietary potassium was associated with increased risk of incident diabetes in African-Americans [19]. Although the unripe plantain+ginger incorporated feed contained higher amounts of K compared with the unripe plantain incorporated feed or standard rat feeds, their consumption by the diabetic rats of this group did not significantly modulate their elevated blood glucose levels compared with the diabetic rats fed unripe plantain incorporated feed alone.
The Recommended Daily Allowance for fat in diabetic patients is 15-20% of the total calorie [20].
Crude fat provided about 12.59% of the total calories of the unripe plantain incorporated feed, 20.44% of the total calories of the unripe plantain+ginger incorporated feed and 7.00% of the total calories of the standard rat feed.
Crude protein provided about 14.33% of the total calories of the unripe plantain incorporated feed, 16.28% of the total calories of the unripe plantain + ginger incorporated feed and 15.05% of the total calories of the standard rat feed.
The Recommended Daily Allowance for proteins in diabetic patients is 15-20% of the total calories [20]. Thus, the range of proteins in the plantain +ginger incorporated feed or the standard rat feeds fell within the recommended range and the implication is that consumption of the unripe plantain +ginger incorporated feed or standard rat feed by the diabetic animals of groups 4 or 2 could meet their daily protein requirement while the protein contents of the unripe plantain incorporated feed did not meet the required standard.
It is worth noting that despite containing large amount of proteins, consumption of the unripe plantain+ginger or standard standard rat feeds by the diabetic rats of group 4 and 2 did not translate into higher weight gain compared with the rats of group 3, suggesting that the higher percentage loss of weight by the diabetic rats of group 2 despite eating highly proteinous diet was as a result of uncontrolled hyperglycemia.
The Recommended Daily Allowance for carbohydrates in diabetics is 50-70% of the total calorie [20]. Thus the range of carbohydrates in the unripe plantain incorporated feed (approximately 55.96% of total calories) was within the recommended range while the range of carbohydrates in the unripe plantain+ginger incorporated feeds (43.12%) and the standard rat feed (approximately 33% of the total calorie) did not meet the required standard.
We therefore conclude that the higher amounts of Mg and Fe in the unripe plantain feeds compared with the unripe plantain+ginger incorporated and standard rat feeds could be one plausible explanation for the higher anti-hyperglycemic action demonstrated by the unripe plantain incorporated feeds compared with the unripe plantain+ginger incorporated feed and standard rat feed.
CONCLUSION
The study showed the potentials of unripe plantain or its combination in the management of diabetes. However, such dietary combination of ginger and unripe plantain at the dosage used in this study was not found to be very effective in the management of diabetes compared with unripe plantain alone. Further studies are therefore recommended to give some detailed explanation regarding this.
CONFLICTS OF INTEREST
The authors declare none
ACKNOWLEDGEMENT
The authors of this manuscript wish to express their profound gratitude to the technical staff of the Food Chemistry Laboratory, Michael Okpara University of Agriculture, Umudike for their assistance during this study and the management of Michael Okpara University of Agriculture, Umudike for sponsoring this study.


