All published articles of this journal are available on ScienceDirect.
The Apoptotic and Antioxidant Effects of Capsaicin on Colorectal Cancer Cell Lines
Abstract
Background
Capsaicin is a natural alkaloid and one of the main active components found in spicy peppers, responsible for their hot taste. It possesses antioxidant and anti-tumor properties.
In this study, the effect of capsaicin on the expression of genes involved in apoptosis, such as Bax, Bcl2, Caspase3, p53, PPARγ, Nrf2, and the activity of antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase, was investigated in LS-180 and HCT-116 human colorectal cancer cell lines.
Methods
Human colorectal cancer cell lines (LS-180 and HCT-116) were treated with various concentrations of capsaicin for 24 hours. The expression levels of genes, including Bax, Bcl2, Caspase3, Nrf2, PPARγ, and p53, were determined using the Real-time PCR method, and the activity of antioxidant enzymes was measured using colorimetric assays.
Results
Analysis of gene expression results showed that capsaicin increased the expression levels of Bax, Bcl2, Caspase3, and p53 in both cell lines, but this increase was statistically significant in the LS-180 cell line. Capsaicin also significantly increased the expression of Nrf2 and PPARγ in both cell lines. The activity of antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase, was increased in both cell lines compared to the control.
Conclusion
It appears that capsaicin may play a role in inducing apoptosis and reducing the proliferation of colorectal cancer cells through the upregulation of Nrf2, PPARγ, and p53 gene expression and the increase in antioxidant enzyme activity. Additionally, the upregulation of Bax and Caspase 3 expression suggests a potential mechanism for capsaicin-induced apoptosis and the reduction of colorectal cancer cell growth.
1. INTRODUCTION
Oxidative stress and the generation of free radicals play a key role in the initiation and progression of various types of cancer [1]. Free radicals, through different metabolic pathways, can influence many cellular processes, such as growth and proliferation, which play a significant role in the onset and advancement of the cancer process [2].
The reduction of the body's antioxidant defense system is considered one of the main factors contributing to the growth and development of cancer [3]. The antioxidant defense system in the body, through enzymatic antioxidants such as superoxide dismutase, catalase, and glutathione peroxidase, as well as non-enzymatic antioxidants such as vitamins E, C, A, flavonoids, metal ion chelators, polyphenolic compounds, and others, weakens the reactions of free radicals [4].
Colorectal cancer is one of the most common cancers worldwide. According to various studies, it appears that oxidative stress may increase the risk of developing colorectal cancer [5].
Capsaicin is a phenolic compound responsible for the spicy taste of red chili peppers. It possesses antioxidant, antimicrobial, and anticancer properties. This compound is used in weight reduction, diabetes control, treatment of metabolic syndrome, pain reduction, and the management of conditions such as leukemia, gastric cancer, prostate cancer, and liver cancer [6].
It seems that capsaicin inhibits the activity of carcinogenic compounds in the body and prevents the formation of malignant tumors [7].
Since the role and mechanism of this substance in this context are not well understood, the present study aimed to investigate the effect of capsaicin on the expression of genes involved in apoptosis and the activity of antioxidant enzymes in LS-180 and HCT-116 colorectal cancer cell lines.
2. MATERIALS AND METHODS
2.1. Cell Culture and Treatment with Hydroxytyrosol
In this study, human colorectal cancer cell lines LS-180 and HCT-116 were obtained from the cell bank of the Pasteur Institute in Tehran. The cells were cultured in DMEM and RPMI media containing 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The cultures were maintained in an incubator at 37 degrees Celsius with 5% CO2 and appropriate humidity. After treating the cells with concentrations of 50, 100, and 200 micromolar of hydroxytyrosol (Sigma-Aldrich) for 24 hours, the cells were collected by trypsinization.
2.2. RNA Extraction and cDNA Synthesis
RNA extraction was performed using the RNA extraction kit (Gena Bioscience, Germany) and the corresponding protocol. To assess the quality and quantity of the extracted RNA, agarose gel electrophoresis (5.1%) and a nanodrop (SpecNano-Arn-20) instrument were utilized. Furthermore, cDNA synthesis was carried out using the cDNA synthesis kit (Gena Bioscience, Germany).
2.3. Evaluation of Gene Expression using Real-time PCR
The expression levels of the genes p53, Nrf2, PPARγ, Bax, Bcl2, and Caspase3 were assessed using the Real-Time PCR technique. The analysis was performed using the Cyber Green master mix (Gena Bioscience, Germany, and Corbett) and the corresponding primers (Table 1) [8].
Fold Change=2−ΔΔCt
ΔCt=Cttarget gene−Ctreference gene
ΔΔCt=ΔCttreatment group−ΔCtcontrol group
2.4. Measurement of Cellular Protein Content
To investigate the activity of antioxidant enzymes, after treatment and cell collection, the protein content of the cells was extracted using a cell lysis buffer, and the total protein amount of each sample was measured using the Bradford method [9].
2.5. Measurement of Antioxidant Enzyme Activity
To assess the activity of the catalase enzyme, a colorimetric method was employed. The measurement was based on the enzyme's reaction with hydrogen peroxide, and the specific activity of the enzyme was calculated in units per milligram of protein [10]. The activity of the superoxide dismutase enzyme was determined by the reaction of superoxide radicals with pyrogallol, and the specific activity of the enzyme was calculated in units per milligram of protein [11].
Measurement of glutathione peroxidase activity was performed based on the colorimetric method and the formation of oxidized glutathione. The specific activity was also calculated in units per milligram of protein [12].
| Gene Name | Sequences | Product Size |
|---|---|---|
| BCL2-F | TCGCCCTGTGGATGACTGA | 134 bp |
| BCL2-R | CAGAGACAGCCAGGAGAAATCA | |
| Bax-F | TGGCAGCTGACATGTTTTCTGAC | 195 bp |
| Bax-R | TCACCCAACCACCCTGGTCTT | |
| Cas3-F | TACCTGTGGCTGTGTATCCG | 134 bp |
| Cas3-R | TCAGTGTTCTCCATGGATACCT | |
| Nrf2-F | ACGGTCCACAGCTCATCAT | 147 bp |
| Nrf2-R | TCCGTCGCTGACTGAAGT | |
| P53-F | GGACATTTGCGTTCGGG | 118 bp |
| P53-R | CTAGGATCTGACTGCGGCTC | |
| PPARγ-F | TAAAGTCCTTCCCGCTGACC | 132 bp |
| PPARγ-R | GGGGTGATGTGTTTGAACTTGA |
2.6. Data Analysis
REST software was used to analyze the obtained data regarding the expression of genes [13]. SPSS software was used to analyze the oxidative stress index and antioxidant enzyme activity. To describe the data, the mean, standard deviation, frequency tables, one-way ANOVA, and Tukey's post hoc test were used.
The study was approved by the ethical committee of Lorestan University of Medical Sciences. (IR.LUMS.REC. 1399.389).
3. RESULTS
3.1. Gene Expression Evaluation
The results of this study showed that the expression level of the Bax gene increased in all doses of capsaicin-treated cells in the two examined cell lines. However, this increase was statistically significant only in the LS-180 cell line (Fig. 1A).
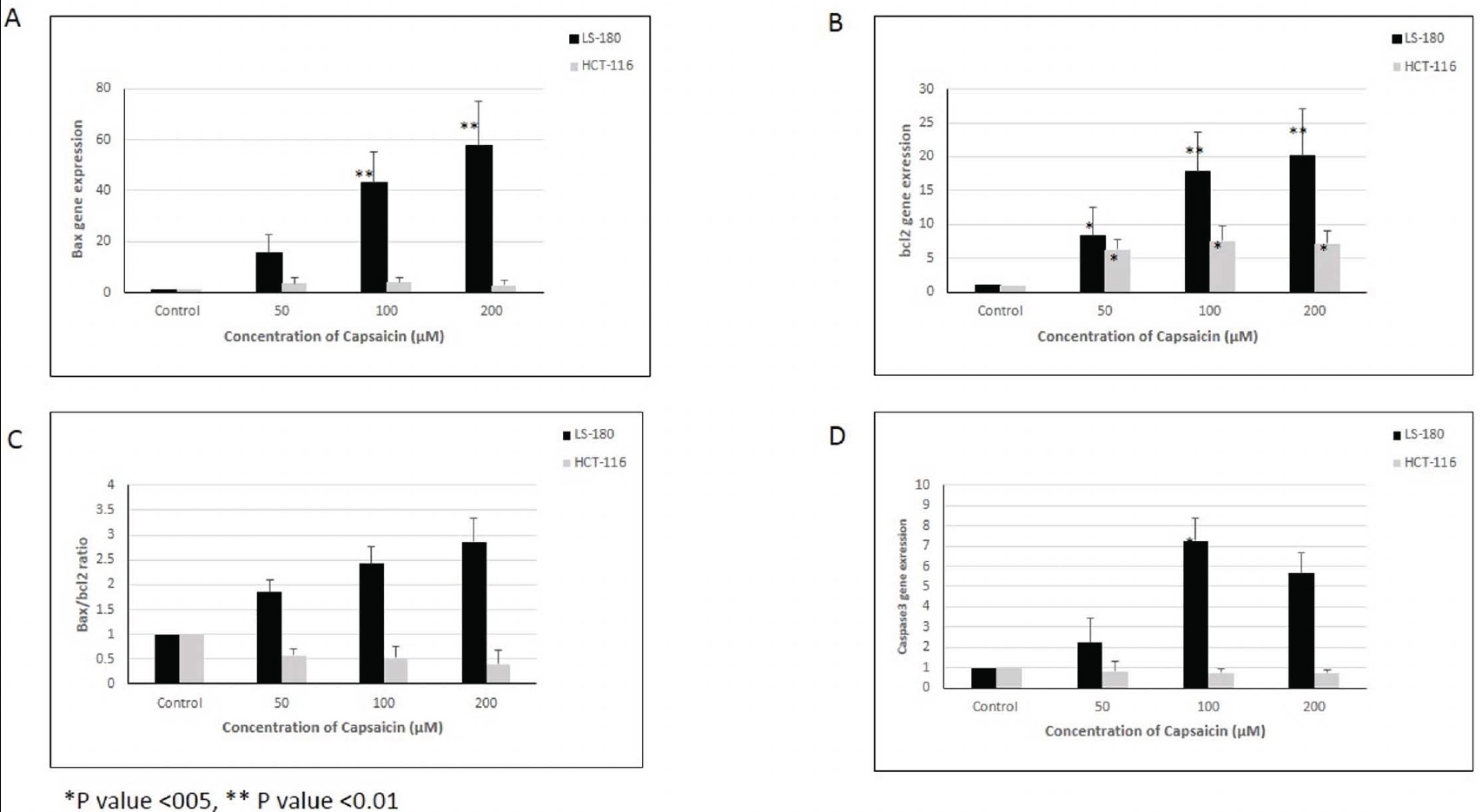
(A) Bax, (B) bcl2, (C) Bax/bcl2 ratio and (D) Caspase 3 gene expression in two colorectal cancer cell lines (LS-180 and HCT-116). *p-value <0.05, **p-value <0.01
The expression level of the Bcl2 gene showed a statistically significant increase in all treated doses in both cell lines compared to the control group (Fig. 1B).
Although the expression levels of the Bax and Bcl2 genes increased in both cell lines compared to the control group, the Bax/Bcl2 ratio showed a statistically significant increase in the LS-180 cell line, while this ratio decreased in the HCT-116 cell line, although the decrease was not statistically significant (Fig. 1C).
The expression level of the Caspase3 gene showed a statistically significant increase in the LS-180 cell line, but no significant change was observed in the HCT-116 cell line (Fig. 1D).
The expression level of the p53 gene increased in both cell lines compared to the control group, and this increase was statistically significant in the LS-180 cell line (Fig. 2A).
The expression level of the PPARγ gene showed a significant increase in both cell lines compared to the control group (Fig. 2B).
The expression level of the Nrf2 gene showed a statistically significant increase in both cell lines compared to the control group (Fig. 2C).
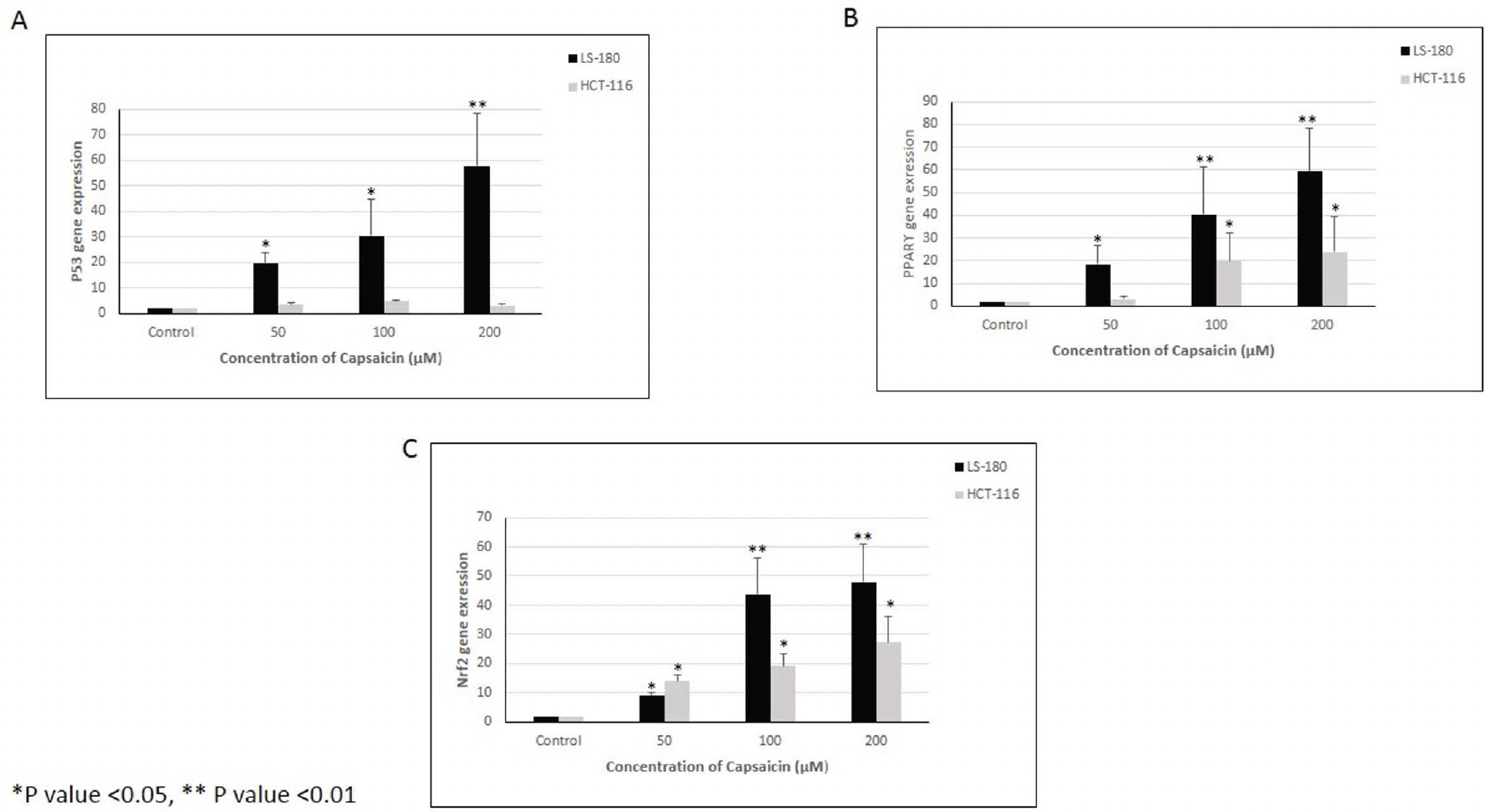
Expression levels of (A) p53, (B) PPARγ, and (C) Nrf2 genes in two colorectal cancer cell lines (LS-180 and HCT-116). *p-value <0.05, **p-value <0.01
|
Enzyme Activity |
Cell Line | Concentration of Capsaicin (µM) | p-value | |||
|---|---|---|---|---|---|---|
| Control (mean±SD) |
50 (mean±SD) |
100 (mean±SD) |
200 (mean±SD) |
|||
|
SOD U/mg protein |
LS-180 | 17.02±1.8 | 22.42±2.6* | 17.1±2.4 | 22.6±2.9* | *p<0.05 |
| HCT-116 | 18.1±1.9 | 29.8±2.2* | 30.1±5.6* | 20.4±2.1* | *p<0.05 | |
|
CAT U/mg protein |
LS-180 | 1.1±0.006 | 2.17±0.8* | 2.12±0.7* | 2.6±0.9* | *p<0.05 |
| HCT-116 | 1.12±0.01 | 2.48±0.2* | 1.97±0.4* | 1.49±0.3 | *p<0.05 | |
|
GPX U/mg protein |
LS-180 | 273.7±14.5 | 297.5±51.2 | 308.1±62.6* | 317.8.4±51.4* | *p<0.05 |
| HCT-116 | 375±44.1 | 371.1.1±43.2 | 416.9±49.9* | 368.1±35.7 | *p<0.05 | |
3.2. Evaluation of Antioxidant Enzyme Activity
Considering the normal distribution of the studied enzyme activity data in this study, a one-way Analysis of Variance (ANOVA) was used to compare the treated groups with different concentrations of capsaicin in the two colorectal cancer cell lines. The results indicate that the mean activity of superoxide dismutase enzyme in both colorectal cancer cell lines showed a statistically significant increase compared to the control group, with a greater increase observed in the HCT-116 cell line compared to the LS-180 cell line (Table 2).
The mean activity of the catalase enzyme also showed an increase in the capsaicin-treated groups in both colorectal cancer cell lines compared to the control group. This increase was statistically significant in the HCT-116 cell line, except for the concentration of 200 μM capsaicin (Table 2).
The mean activity of the glutathione peroxidase enzyme also showed an increase in both cell lines compared to the control group. This increase was statistically significant at concentrations of 100 and 200 μM in the LS-180 cell line and at a concentration of 100 μM in the HCT-116 cell line (Table 2).
4. DISCUSSION
The use of natural compounds and food derivatives has attracted significant attention from researchers due to their therapeutic effects and fewer side effects [14]. Capsaicin (N-vanillyl-8-methyl-1-nonenamide) is a natural alkaloid and one of the main components of hot peppers. Various studies have shown the apoptotic effect of capsaicin in different cancer cells, such as gastric, colon, prostate, etc., without exerting cytotoxic effects on normal cells [7, 15-17].
Capsaicin has a high affinity for vanilloid subtype 1 (TRPV1) and vanilloid subtype 6 (TRPV6) receptors, which belong to the Transient Receptor Potential (TRP) superfamily of nonselective cation channels. The expression of these receptors generally increases in cancer cells compared to normal and healthy cells [17-23].
It has been shown that the binding of capsaicin to TRPV1 leads to the activation of various mechanisms, including the generation of reactive oxygen species such as superoxide radicals and hydrogen peroxide, as well as an increase in intracellular calcium levels. This results in an increase in the levels of Bax, GADD153, and GRP78, a decrease in mitochondrial membrane potential (mΨΔ), and the expression of Bcl-2, xIAP, and cIAP1, and subsequently, the activation of caspase-3, leading to apoptosis induction in HepG2 cells [15]. In addition to apoptosis induction, capsaicin has been observed to induce cell cycle arrest in human cancer cells [24].
Various studies have shown that capsaicin induces G1 cell cycle arrest in CE 81T/VGH human epidermoid carcinoma cells and prostate cancer cells through the induction of p53 and cyclin-dependent kinase (cdk) inhibitor p21 [25-29]. Treatment of HL-60 human leukemic cells also leads to G1 arrest through the inhibition of cdk2 activity.
The anti-angiogenic activity of capsaicin is also associated with its ability to induce G1 arrest in endothelial cells. G1 arrest is achieved by reducing the levels of cyclin D1, inhibiting cdk4 activity, and phosphorylating Rb in endothelial and breast cancer cells [30]. These findings suggest the potential anti-proliferative activity of capsaicin through the E2F-Rb pathway.
Furthermore, it has been shown that the anti-proliferative effects of capsaicin in Small Cell Lung Cancer (SCLC) are mediated through the reduction of the expression of proliferative genes responsive to E2F, such as cyclin E, thymidylate synthase, cdc25A, and cdc6 [24].
Researchers have demonstrated that capsaicin plays an important role in inducing apoptosis in cells through the generation of oxidative stress [15]. It appears that capsaicin inhibits complexes I and III of the mitochondrial electron transport chain and disrupts the electron flow in the mitochondrial membrane, leading to the competition with coenzyme Q, the production of reactive oxygen species, and the creation of a prooxidant environment in the plasma membrane [31]. Since the concentration of free oxygen radicals is higher in cancer cells compared to normal cells, cancer cells may be more sensitive to the production of active oxygen species, and an increase in limited amounts of free radicals can lead to apoptosis induction in these cells [32].
Furthermore, various studies have shown that oxidative stress influences many cell proliferation-related signaling pathways, including the Epidermal Growth Factor Receptor (EGFR) signaling pathway, in which proteins such as nuclear factor erythroid 2-related factor 2 (Nrf2) and Raf are involved.
In addition, oxidative stress affects Mitogen-Activated Protein Kinases (MAPKs), Phosphatidylinositol 3-Kinase (PI3K), phospholipase C, and protein kinase C. Moreover, reactive oxygen species influence the tumor suppressor gene p53 involved in programmed cell death [33-36].
It has been shown that capsaicin activates AMP-activated protein kinase (AMPK) in HepG2 cells through binding to the TRPV1 receptor, increasing cytosolic calcium levels, and activating calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ). AMPK activation, through the generation of reactive oxygen species and inhibition of Akt/mTOR, leads to decreased cell survival and induction of apoptosis [14].
Furthermore, it appears that capsaicin binding to TRPV6, through increasing cytosolic calcium levels and activating apoptotic regulatory factors such as calpains and calcium-dependent cysteine proteases, induces apoptosis in Small Cell Lung Cancer (SCLC) cells [22].
The results of the current study demonstrate the effect of capsaicin in increasing the activity of antioxidant enzymes, including superoxide dismutase, catalase, glutathione peroxidase, as well as the expression of genes such as Bax, caspase-3, p53, PPARγ, and Nrf2 in colorectal cancer cell lines. However, these changes were more pronounced in the LS-180 cell line compared to the HCT-116 cell line.
It appears that these changes may be effective in inhibiting growth and inducing apoptosis in colorectal cancer cell lines through various mechanisms.
Since various studies have shown the impact of capsaicin on the generation of reactive oxygen species [14, 15], the production of reactive oxygen species by capsaicin likely leads to an increase in the expression of Nrf2, followed by PPARγ.
As studies have shown, there is a mutual relationship between the expression of these two factors, and an increase in the expression of PPARγ can lead to an increase in the expression of Nrf2 [36]. The upregulation of these factors plays a crucial role in increasing the expression and activity of antioxidant enzymes [37].
Moreover, an increase in PPARγ expression can induce the expression of genes such as Bax, caspase-3, and p53 [38-40]. On the other hand, Reactive Oxygen Species (ROS) also play a role in inducing the expression of p53.
Although p53 is a tumor suppressor and plays a crucial role in inhibiting carcinogenesis, it has been shown that this factor also contributes to mitochondrial respiration impairment, increased production of Reactive Oxygen Species (ROS), and induction of oxidative stress. Furthermore, this factor is involved in increasing the expression and activity of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase [41-43].
On the other hand, this factor, by binding to the anti-apoptotic protein Bcl-2, counteracts its inhibitory effect on Bax, leading to increased mitochondrial membrane permeability and release of cytochrome C. Additionally, this factor plays a role in increasing the expression of genes involved in apoptosis, such as Bax and caspase-3 [44, 45].
CONCLUSION
Based on the findings of the present study, capsaicin appears to induce oxidative stress and upregulate the expression of Nrf2, PPARγ, and p53 genes. This leads to increased antioxidant enzyme activity and the expression of genes involved in apoptosis, contributing to the induction of apoptosis and inhibition of colorectal cancer cell proliferation. The expression level of the Bax gene increased in capsaicin-treated LS-180 cells, the same as the effect of capsaicin on the p53 gene. The same phenomena occur in the conclusions of capsaicin's effects on enzymatic activities.
This suggests that, in addition to the concentration and duration of capsaicin treatment, the inherent characteristics of cancer cells may influence their response to capsaicin. To confirm these results, additional assays such as flow cytometry for apoptosis (e.g., annexin V versus propidium iodide) and cell proliferation assays (e.g., MTT, MTS, CCK-8) are recommended.
AUTHORS’ CONTRIBUTION
M.H.: Conceptualized and designed the study, designed the data collection instruments, collected data, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript; P.B.: Coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content.
LIST OF ABBREVIATIONS
| TRP | = Transient Receptor Potential |
| SCLC | = Small Cell Lung Cancer |
| EGFR | = Epidermal Growth Factor Receptor |
| MAPKs | = Mitogen-Activated Protein Kinases |
| PI3K | = Phosphatidylinositol 3-Kinase |
| AMPK | = Activated Protein Kinase |
| SCLC | = Small Cell Lung Cancer |
| ROS | = Reactive Oxygen Species |
| CO2 | = Carbon Dioxide |
ETHICAL STATEMENT
The study was approved by the ethical committee of Lorestan University of Medical Sciences, Iran (IR.LUMS.REC.1399.389).
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


