Targeting of Hypoxia for Therapeutic Strategy in the Varied Physiological States
Abstract
Hypoxia-inducible factors (HIFs) are transcription factors that initiate the expression of cellular processes to cope with hypoxic conditions. HIFs are principal regulators of hypoxic adaptation, regulating gene expression involved in glycolysis, erythropoiesis, angiogenesis, proliferation, and stem cell function under low O2. HIFs may play a pivotal role in tumor survival and metastasis in cancer formation and growth. Likewise, HIFs play a key role in microbial pathogenesis, particularly in host-pathogen interaction. Because of the role that HIF-1alpha plays in the biology of cancer and infections, it is a potential therapeutic target not only for malignant growth but also for parasitic infection. Several reports have demonstrated the up-regulation of host cellular HIFs due to infection-induced hypoxia. Hypoxia-inducible pathways have attracted great interest for treating inflammatory diseases and infections by viruses, protozoa, or bacteria, among other pathogens. Interestingly, increasing evidence suggests that HIFs play an important regulatory role in inflammation. For example, in macrophages, HIFs regulate glycolytic energy generation and optimize innate immunity, control pro-inflammatory gene expression, mediate the killing of pathogens and influence cell migration. Therefore, a good understanding of the biochemical mechanism of hypoxia signaling pathways will shed more light on how it could help identify and develop new treatment strategies for cancer and parasitic diseases, including viral, bacterial, fungal and protozoa infections.
1. INTRODUCTION
Typically, homeostasis mechanisms regulate tissue O2 concentrations at the cellular, organ, and systemic levels. Nearly all eukaryotes need oxygen for various metabolic reactions [1]. A deficit in oxygen supply or excessive oxygen usage could lead to a shortage in oxygen levels required to maintain cellular function, a state regarded as hypoxia [2]. Hypoxia is the shortage of oxygen reaching the organs and tissues and is a significant factor in the pathology of dozens of human diseases such as cancer, diabetes, stroke, and many other parasitic diseases [2, 3]. Hypoxia can occur due to inadequate oxygen reserve due to low blood flow to the tissues or a small amount of oxygen in the blood (hypoxemia) [4, 5]. The insufficient supply of oxygen to tissues decreases energy availability to the tissues and triggers transcriptional responses, which allow tissues to reduce their oxygen intake and adjust to the hypoxic conditions [6, 7]. Hypoxia varies in severity from mild to drastic [8]. There are variations in responses to hypoxia; whereas many tissues can tolerate some forms of hypoxia for an extended period, others may not [9].
Adaptation to low oxygen levels (hypoxia) within tissues and cells necessitates the activation of numerous genes involved in iron and glucose metabolism, cell proliferation and survival, and angiogenesis. HIF-1 is a vital transcriptional intermediary in hypoxic reactions and a major oxygen homeostasis regulator in eukaryotic cells [10]. Initially, HIF-1 was described as a transcription factor expressed due to low levels of blood oxygen by regulating EPO expression [11]. HIF-1 consists of two diverse subunits, the alpha subunit and the beta subunit. The beta subunit is also referred to as an aryl hydrocarbon nuclear receptor translocator (ARNT); the subunits are in the family of fundamental helix-loop-helix Per-Arnt-Sim (bHLH-PAS) transcription factors [12]. During persistent hypoxic exposure, expression of HIF-1 occurs when there is no equilibrium between the amount of oxygen transported and the amount utilized by the tissue [12]. In situations when oxygen pressure is adequate, a family of prolyl hydroxylase enzymes (PHDs) hydroxylates de novo-produced cytoplasmic HIF-1 molecules in ODDD (oxygen-dependent degradation domain) located on prolines 402 and 564 [13].
Hypoxia-inducible factors (HIFs) are transcriptional factors caused by hypoxic conditions which react to a decline in oxygen levels [14, 15]. HIF-1 is necessary for immunological reactions and is vital for the physiological regulation of homeostasis, vascularization, and anaerobic metabolism. Moreover, the investigation of HIF-1 is progressively expanding due to its potential therapeutic prospect. HIF-1alpha inhibition may help to prevent carcinogenesis by depriving tumor cells of oxygen and nutrients [15]. Interestingly, increasing evidence suggests that HIFs play an important regulatory role in inflammation. For example, in macrophages, HIFs regulate glycolytic energy generation and optimize innate immunity, control pro-inflammatory gene expression, mediate the killing of pathogens and influence cell migration. Also, HIF-1alpha might be a physiological feature that potentially targets parasitic infection. Several reports have demonstrated the up-regulation of host cellular HIFs due to infection-induced hypoxia [16]. Hypoxia-inducible pathways have attracted great interest in the down-regulation of prolyl hydroxylase for treating inflammatory diseases and infections by viruses, protozoa, or bacteria, among other pathogens. Therefore, a good understanding of the biochemical mechanism of hypoxia signaling pathways will shed more light on how it could help identify and develop new treatment strategies for cancer and parasitic diseases, including viral, bacterial, fungal and protozoa infections.
2. HYPOXIA
Hypoxia denotes a low level of oxygen or deprivation in the quantity of oxygen that reaches the tissues of an organism’s body. Hypoxemia, which describes an insufficient quantity of oxygen in the blood, may lead to hypoxia. For instance, if an insufficient amount of oxygen in the blood occurs, it often leads to insufficient oxygen in the tissues or may be due to other effects. The origin of hypoxia has two major classifications: those seen as a decrease in oxygen blood content or oxygen blood supply. However, an exceptional example of carbon monoxide (CO) poisoning may happen due to a combination of these two types. Hypoxia might result from inadequate oxygen levels within the blood circulating a tissue. On the other hand, arterial blood might indicate a common oxygen strain but might not be capable of carrying the necessary amount of oxygen when minute hemoglobin in the blood is present, as it may arise in anemia. Perhaps, it may be useful at this point to differentiate between “hypoxemia” and “hypoxia.” Hypoxia occurs due to reduced oxygen transport to tissues, while hypoxemia occurs due to decreased oxygen strain in arterial blood. Hypoxia can still occur even in environments with normal blood oxygenation when the blood does not perfuse the tissues at an acceptable level. Reduced blood tissue perfusion will result in a significant decrease in tissue oxygenation. Such cases frequently occur in conditions of shock when rapid heart failure reduces the flow of well-oxygenated blood.
The symptoms of hypoxia vary among individuals and depend on the severity of the hypoxia. Some of these symptoms include syncope, dyspnea, confusion, headache, tachycardia, increased respiratory rate, euphoria, tingling sensations, elevated blood pressure, lack of organization, and visual alterations [17]. Hypoxia occurs for several reasons based on insufficient oxygen, and these include hypoxic hypoxia, metabolic hypoxia, histotoxic hypoxia, stagnant hypoxia, and anemic hypoxia [17-19].
Adaptation to low oxygen levels (hypoxia) within tissues and cells necessitates the activation of numerous genes involved in iron and glucose metabolism, cell proliferation and survival, and angiogenesis. HIF-1 is a vital transcriptional intermediary in hypoxic reactions and a major oxygen homeostasis regulator in eukaryotic cells [10]. Initially, HIF-1 was described as a transcription factor expressed due to low levels of blood oxygen by regulating EPO expression [11]. HIF-1 consists of two diverse subunits, the alpha subunit and the beta subunit. The beta subunit is also referred to as an aryl hydrocarbon nuclear receptor translocator (ARNT); the subunits are in the family of fundamental helix-loop-helix Per-Arnt-Sim (bHLH-PAS) transcription factors [12]. During persistent hypoxic exposure, expression of HIF-1 occurs when there is no equilibrium between the amount of oxygen transported and the amount utilized by the tissue [12]. In situations when oxygen pressure is adequate, a family of prolyl hydroxylase enzymes (PHDs) hydroxylates de novo-produced cytoplasmic HIF-1 molecules in ODDD (oxygen-dependent degradation domain) located on prolines 402 and 564 [13].
2.1. Hypoxia-Inducible Factors (HIFs)
HIFs are transcriptional activators that majorly regulate oxygen homeostasis in different organisms, most often in connection to adaptations to high altitudes and other oxygen-deficient environments [20, 21]. In humans, HIFs consist of three HIF -subunit paralogs (HIF-1, HIF-2, and HIF-3) and two HIF-subunit paralogs (aryl hydrocarbon nuclear translocator; ARNT, and ARNT2). Furthermore, the operational HIF transcription factor complex may result from heterodimerization of HIF- subunits [22, 23]. In diverse organisms, the hypoxic decreasing partial pressure of oxygen controls the regulative HIF -subunit, whereas, in normoxic situations, it leads to its breakdown; hence, HIF activity is regulated at the -subunit level [24].
HIF and HIF (ARNT) genes belong to the bHLH+PAS-containing gene family, and their proteins are different by the presence of an N-terminal bHLH DNA-binding domain upstream of two PAS domains [25]. Furthermore, subunits comprise an inhibitory domain known as the oxygen-dependent degradation domain (ODDD) and an N-terminal transactivation domain (NTAD) [26]. HIF-1 and HIF-2, subsets of HIF-1 proteins, are different by the presence of a C-terminal transactivation domain (CTAD) at the protein's C-terminus [23]. These domains are significant in the function of HIF proteins. The bHLH domain makes contact with the core nucleotides of HIF-responsive elements while, at the same time, the bHLH and PAS domains in synergy mediate both dimerization and sequence-specific DNA binding [27, 28].
2.2. Hypoxia Inducible Factor 1 (HIF-1)
HIF-1 is a heterodimer protein made up of two subunits: HIF-1alpha and HIF-1beta transcriptional factors [29]. The HIF-1 transcription factor is an essential controller of the translational response [30]. HIF prolyl hydroxylases (PHDs) closely control the stability of HIF-1α expression [31].
Hypoxia-inducible factor 1 dimeric proteins are important in response to the lowered oxygen concentration in the body. One of the primary genes involved in homeostasis regulation that often increases vascularization in ischemia and tumor cells (hypoxic environment) is HIF-1. Hypoxia-inducible factor 1 is a transcription factor needed for several target genes; HIF-1 is necessary for immunological reactions and is vital for the physiological regulation of homeostasis, vascularization, and anaerobic metabolism. Moreover, the investigation of HIF-1 is progressively expanding due to its potential therapeutic prospect. The function of angiogenesis improves the gene in ischemic patients and may stimulate the proliferation of vessels required for oxygenation.
Reduced PHD and FIH activities reduce HIF 1alpha in hypoxia, causing it to translocate to the nucleus, forming a complex and binding to HIF 1beta. This complex binds target genes with the hypoxia-responsive element (Fig. 1) [31].
2.3. HIF-1 Functions
Hypoxia-inducible factor 1, a key regulator of cellular oxygen homeostasis, regulates and affects the genetic expression associated with sustaining homeostasis as the concentration of oxygen is altered [21]. It also regulates glucose uptake and facilitates anaerobic respiration in hypoxic environments [32]. Additionally, HIF-1 is a significant inflammation regulator connected to the regulation of both acute and chronic inflammation [33].
HIF-1 significantly stimulates angiogenesis and controls the movement of mature endothelial cells during hypoxic conditions [32]. Then, the regulation of vascular endothelial growth factor (VEGF) transcription by HIF-1 completes the reaction. In hypoxic conditions, transcription and expression of the VEGF gene occur once HIF-1 binds to its regulatory region [34]. These endothelial cells form new blood vessels that supply oxygenated blood [35].
In hypoxic environments, HIF-1 regulates anaerobic metabolism through alteration in the production of cellular energy. Hypoxia-inducible factor 1 helps this shift by inducing varieties of glucose transporters and glycolytic enzymes (pyruvate kinase M and aldolase A) that assist cells inefficient energy production in hypoxic environments [36]. In addition to the increase in enzyme expression, HIF-1 reduces mitochondrial oxygen depletion by the activation of pyruvate dehydrogenase kinase I and inhibits the tricarboxylic acid cycle (TCA) [37].
Natural hypoxia exists in tumor environments where metastasizing tumor cells proliferate.HIF-1 induces an alternative metabolic pathway in the tumor microenvironment, making it an essential protein in tumor masses.
3. CELLULAR RESPONSES TO HYPOXIA
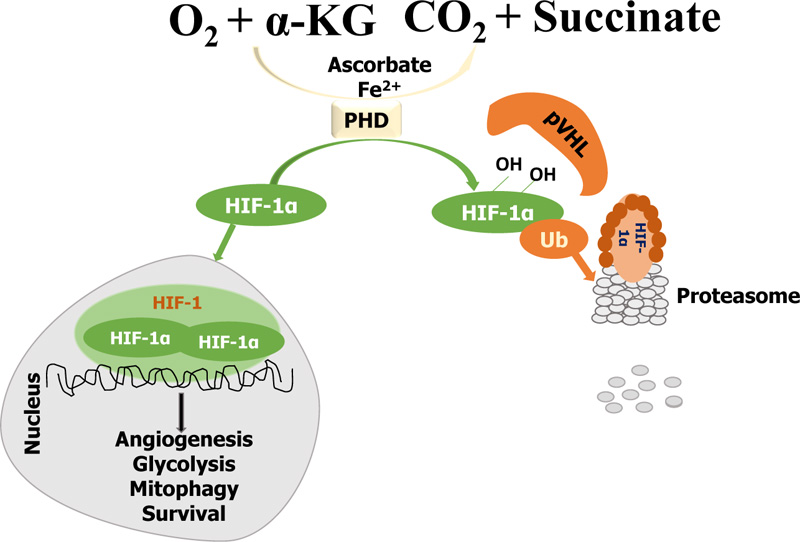
When the oxygen level in the blood falls, the body undergoes responses, including increased respiration and blood flow. At the same time, individual cells experiencing hypoxia start to react to the decrease in oxygen tension. They sense low oxygen through cellular signaling that begins with prolyl hydroxylase domain (PHD) proteins, a class of enzymes with evolutionarily conserved oxygen-, iron-, and alpha-ketoglutarate-dependent dioxygenase that can catalyze the hydroxylation reaction of specific proline residues on HIF-1 (Fig. 1) [38, 39]. Apart from PHD proteins, factor inhibiting HIF (FIH), belonging to the 2-oxoglutarate-dependent dioxygenase family, also regulates HIF [40].
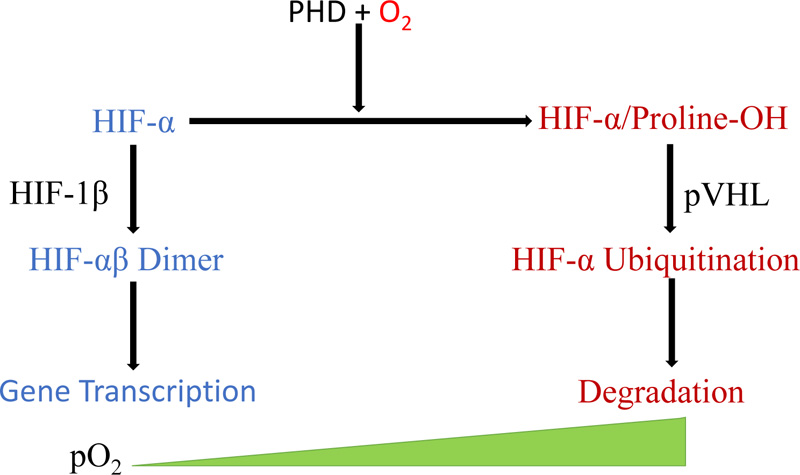
All nucleated cells of metazoans, including humans, express hypoxia-inducible factors. The activation of HIF occurs under a low oxygen tension and leads to the regulation of specific genes. In the presence of low oxygen levels, PHD cannot destroy HIF, which then enters the nucleus and modulates transcription [2]. Hypoxia-inducible factor binds directly to promoter sequences in DNA, increasing the transcription rates of specific hypoxic response genes [23]. This binding consequently leads to changes in glucose metabolism from aerobic to anaerobic, increased cytochrome oxidase transcription, inhibition of lipid catabolism, and promotion of lipid storage [2].
Through this regulation of gene expression, HIF can suppress oxidative metabolism and switch to glycolytic metabolism [41]. The enzymes of the glycolytic cycle, e.g., lactate dehydrogenase A (LDHA) and pyruvate dehydrogenase kinase 1 (PDK1), are directly upregulated by the activities of HIF-related increases in transcription, whereas those that use acetyl-CoA in the tricarboxylic acid (TCA) cycle for oxidative phosphorylation are shut down indirectly by HIF [41-44]. The upregulated enzymes then inhibit the generation of acetyl-CoA from pyruvate and, as a result, inhibit the generation of ATP from oxidative phosphorylation. Accordingly, the pyruvate formed from glycolysis forms lactate. This prevents pyruvate from fueling the mitochondrial TCA cycle, thus reducing mitochondrial oxygen consumption and preventing increased production of reactive oxygen species (ROS) [44, 45].
As hypoxia is established, the function of the electron transport chain in mitochondria wanes because electrons leak out of the mitochondria before getting to cytochrome c oxidase (COX) [46, 47]. With the mitochondria not functioning properly, the cell stops sending acetyl-CoA to the TCA in order not to overwhelm the mitochondria. Rather, the cell focuses on glucose utilization for fuel to eliminate lactate, a by-product of anaerobic respiration [2]. Thus, HIF signals the cell to switch to anaerobic metabolism from aerobic metabolism.
One of the important molecular responses to hypoxia is stabilizing the hypoxia-inducible factor (HIF)-1a, enabling interaction with HIF-1b. The complex translocates to the nucleus, where it binds to hypoxia-responsive elements (HRE) in the promoter region of target genes, such as vascular endothelial growth factor (VEGF), glucose transporters 1 (GLUT-1), and carbonic anhydrase IX (CAIX) [35]. CAIX and other membrane transporters, like the sodium-proton exchanger 1 (NHE-1) and the monocarboxylate transporters (MCT), are upregulated to counteract the hypoxia-induced intracellular acidosis. CAIX is a tumor-specific dimeric membrane-bound zinc metalloenzyme, which catalyzes the reversible hydration of carbon dioxide to bicarbonate and a proton to help maintain the cell's pH homeostasis [36]. High tumoural CAIX expression has been associated with poor prognosis, tumor progression, and aggressiveness [37]. Inhibition of its function would therefore be a promising anticancer approach to target the hypoxic compartment of tumors.
4. REGULATION OF HIF
Regulation of HIF occurs through two oxygen-dependent mechanisms: PHD represents one part of the regulation of HIF, and FIH represents the other. Essentially, the enzymes of the families of both PHD and FIH utilize ascorbate, a-ketoglutarate (alpha-KG), and O2 as their substrates, whereas their catalytic center consists of Fe2+ [48]. The role of PHD is to bind specific residues of HIF-1alpha or HIF-2alpha subunits and cause ubiquitination, leading to the degradation of HIF in the proteasome and, as a result, preventing HIF from being involved in gene transcription. On the other hand, FIH prevents the activation of HIF by blocking the co-activators of HIF. At low oxygen levels, oxygen serves as a limiting substrate for both FIH and PHD; when the oxygen levels are normal, PHD and FIH are active but inactive when the oxygen levels are low [2, 25].
In normal levels of oxygen (normoxia), PHD hydroxylates HIF-1alpha on the proline residues 402 and 564 within the oxygen-dependent degradation domain, activating von Hippel Lindau tumor suppressor protein (pVHL)-mediated ubiquitination and proteasomal degradation [49-51]. The prolyl hydroxylation on the oxygen-dependent degradation (ODD) domain of HIF-1alpha allows the recruitment of pVHL, which acts as a substrate recognition of the E3-ubiquitin ligase protein complex [52]. The hypoxia-inducible factor degradation occurs in the proteasome, and the cell continues aerobic respiration. Simultaneously, FIH, an asparaginyl hydroxylase, targets asparaginyl residues on the C-terminal transcriptional activation domain (CAD) of HIF subunits (HIF-1alpha) and represses transcriptional activity of HIF by blocking the co-activator p300/CBP recruitment to CAD [40, 49, 53]. The hypoxia-inducible factor needs the binding of the co-activators p300 and CBP to be transcriptionally active, while P300 and CBP require asparaginyl residues to recognize and trigger HIF. Therefore, when FIH hydroxylates the asparaginyl residues, the co-activators do not interact with HIF, thereby blocking it from becoming transcriptionally active [54].
In hypoxia, on the contrary, the prolyl hydroxylation is prevented due to the inadequate supply of oxygen needed for the catalytic activity of the PHD (PHDs are inhibited in the process), leading to the accumulation and stabilization of HIF-1alpha. Usually, HIF-1alpha undergoes post-translational modifications during hypoxia, particularly phosphorylation, which can regulate its stability [55, 56]. Afterward, the stabilized HIF-1alpha is transferred into the nucleus and dimerized with HIF-1beta, thereby creating an active HIF-1 complex and activating the transcription of genes that promote glycolysis, angiogenesis, mitophagy, and survival (Fig. 2) [57, 58].
Several other means of regulating hypoxia-inducible factors are:
(a) Muscle A-kinase anchoring protein (mAKAP): They are scaffolding proteins responsible for multi-protein complexes' assembly. MAKAPs organize the E3 ubiquitin ligase complex, which affects HIF positioning and stability in the enzyme active site. Decreased mAKAP would alter the stability of the HIF complex.
(b) Dimethyloxalylglycine (DMOG) is an inhibitor of alpha-ketoglutarate, and it would abrogate hydroxylase function, to enhance the transcription of HIF [59].
(c) Iron chelating agents such as desferrioxamine and cobalt chloride are HIF activators [60]. The chelation of iron ions present at the catalytic centers inhibits hydroxylase enzymes.
4.1. Hypoxia Inducible Factor 2 (HIF-2)
Hypoxia-inducible factor 2 is an endothelial per-ARNT-sim domain protein 1 (EPAS1) found in the interstitial and parenchyma cells of numerous organs, including astrocytes, hepatocytes, cardiomyocytes, duodenum, lung, heart, etc. initially, hypoxia-inducible factor 1 was first identified, but both are involved in cancer metastasis, angiogenesis, and cell differentiation. It is the key regulator of physiological and pathophysiological angiogenesis; atthe minimum, it is equally significant as HIF-1alpha [43, 62]. Certainly, HIF-2alpha controls various aspects of angiogenesis, and the growth of blood vessels, metastasis, cell proliferation, and movement [63]. In normoxic conditions, asparaginyl hydroxylation represses the transactivation activity of hypoxia-inducible factor 2alpha [43]. Hypoxia-inducible factor 2 might be absent in early tumor lesions but is constantly synthesized in overt carcinomas and solid tumors. It has also been shown to actively regulate genes that participate in tumorigenesis and cancer development [43, 62].
4.2. Hypoxia and Cancer: Hypoxia-Inducible Factor-1, Tumor Responses, and Targeted Treatment Prospects
In tumor cells, the level of oxygen present in the tumor microenvironment is significantly reduced. The solid tumor shows partial pressure (PO2) of less than 10 mm compared with the normal tissue (45-65 mm). There is insufficient blood perfusion in severe or temporary hypoxia, while prolonged hypoxia limits the spread of oxygen in inflamed tumors. This resulted in the activation of HIF-1 and HIF-2 and linked HIF-1alpha over-expression to mortality and metastasis [32]. HIF-1 alpha is overly expressed in human cancer cells, but it depends on the cancer type. The overexpression of HIF-1alpha has led to high patient mortality rates from cancers of the cervix, brain, uterus, breast, ovary, and oropharynx, whereas the overexpression of HIF-1 alpha may be linked with reduced mortality in neck and head cancer patients [64]. Studies reveal that HIF-1alpha assists chemotherapy and radiation resistance. HIF-1alpha inhibition may help to prevent carcinogenesis by depriving tumor cells of oxygen and nutrients [42].
4.3. HIFs in Cancer Progression
Studies have shown how important HIFs are in different phases of cancer cell initiation and progression. The different phases in cancer cell development include epithelial-mesenchymal transition, metastasis, metabolic reprogramming, angiogenesis, invasion, and proliferation and survival of the cell (Fig. 3). Through different research launching HIF as a cancer therapy target, HIF-1alpha and HIF-2alpha levels are involved with tumor growth, vascularization, and metastasis in animal and clinical-based studies. Additional highlights on some HIF-regulated gene expressions essential in cancer development [25] are:
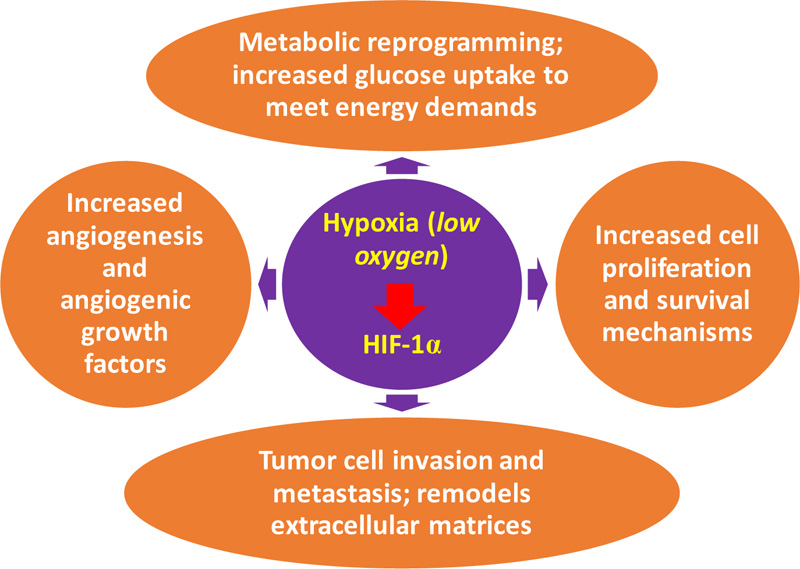
(a) Increased cell proliferation and survival: This is the primary distinction between normal and tumor cells, and it is due to autocrine signaling. This signaling enhanced the proliferation and survival of cells. The ATP level is an essential cellular apoptotic determinant, as profuse glycolytic ATP results in apoptosis throughout hypoxia.
(b) Metabolic reprogramming: In cancerous cells, glucose uptake increased greatly to meet cell energy demands. This is the basis of imaging with 18-F-fluorodeoxyglucose-positron emission tomography (FDG-PET) for identifying metastases. The tumor-related metabolic switch HIF-1 moderates the Warburg-related effect, leading to greater glucose oxidation under anaerobic conditions than oxidative phosphorylation. Tumour microenvironment acidosis is the critical effect of this shift. The acidic and metabolic environments are responsible for abundant metabolic intermediates that encourage tumor progression and aggression [65].
(c) Angiogenesis: In response to reduced oxygen, particularly within a cancer cell, the formation of new capillaries from existing vessels aids oxygen transport to cancerous cells and thus boosts the growth of tumors [66]. The hypoxia-inducible factor-1’sangiogenic switch could be linked with increased oxygen consumption in the hypoxic tumor microenvironment while the diffusion of oxygen decreases. Angiogenesis is a complicated, well-ordered process necessary for the development of neoplasms. The mechanism includes a large number of genes, pathways, and regulators. Angiogenesis leads to increased vascular density and low diffusion of oxygen [67]. Hypoxia-inducible factor 1 also regulates angiogenic growth factor expression of the encoding genes. These include angiopoietin 1 and 2, stromal-derived factor-1 (SDF-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor B (PDGFB), and placenta growth factor (PGF) [25].
(d) Tumor cell invasion and metastasis: Tumor cell invasion and metastasis regulate hypoxia. Metastasis is the core reason for a series of well-defined events in cancer mortality. These events include survival of circulating tumor cells and extravasation, local cell propagation, intravasation, and colonization. Hypoxia-inducible factor-regulated gene activation could enhance multi-tumor metastases. Hypoxia-inducible factor 1 stimulates genetic transcription such as metalloproteinases 2, 9, and 14, cathepsin C degradation (CTSC), and remodels lysyl oxidase (LOX) or plasminogen urokinase activator, the extra-cellular matrices within metastasis [67].
4.4. HIF-1 Inhibitors in Cancer Therapy
Several drugs have been proposed to stop or inhibit HIF activity via multiple molecular mechanisms. Most of these compounds act by reducing HIF-1alpha protein synthesis, mRNA level expression, and transcriptional activity. They also act by increasing HIF-1alpha degradation and decreasing the rate at which DNA binds to HIF [25]. The decrease in HIF-1 mRNA expression: HIF-1 inhibitors target pathways involved in the translation or degradation of protein because these pathways regulate increased HIF-1. However, the mRNA levels of HIF-1 can affect its protein translation in hypoxic conditions [68]. An agent affecting the HIF-1 mRNA is aminoflavone (AF), acting as an aryl hydrocarbon receptor ligand (AhR) and is currently used in clinical studies for patients with metastatic cancer [69, 70].
4.4.1. Inhibition of HIF-1 Protein Synthesis
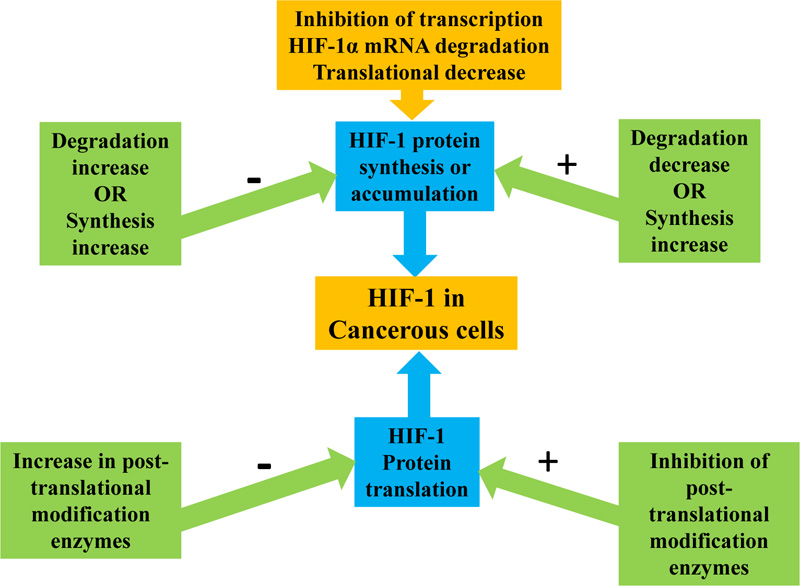
Agents that affect the synthesis of HIF-1 protein include inhibitors of cyclin-dependent kinase, topoisomerase I and II, tumor formation pathway, thioredoxin reductase, and tyrosine kinase. The activation of HIF-1 is highly dependent on the synthesis and HIF-1 protein availability. Therefore, to deactivate HIF-1, some compounds act by inhibiting or decreasing its protein synthesis [71] and regulating the rate of synthesis and degradation to control HIF-1alpha protein accumulation. A decrease in protein synthesis or an increase in its degradation prevents HIF-1alpha protein accumulation, while a decrease in degradation or an increase in synthesis can induce HIF-1alpha protein (Fig. 4). The major mechanisms of decreasing HIF-1alpha protein synthesis are HIF-1alpha mRNA degradation and translational decrease [71]. HIF-1alpha protein synthesis is inhibited by microtubule disrupting agents (MDA) by preventing the activation of HIF-1 and accumulation of HIF-1alpha protein. Masoud, G. N. and Li, W [65]. describe an RNA module consisting of synthetic oligonucleotide antisense that specifically binds and inhibits HIF-1alpha mRNA expression [65]. HIF-1 alpha mRNA has been downregulated in doses dependent upon binding to EZN-2968, resulting in both normoxia and hypoxia inhibition [72]. The EZN-2968 therapy showed tumor reduction in mice implanted with DU-145 human prostate cancer cells. The evaluation in 4 out of 6 patients with paired biopsies of the patient's EZN-2968 therapy showed that HIF-1alpha mRNA was decreased in post-treatment biopsies, while y, 2 patients had reduced mRNA level, and in target gene protein HIF-1alpha [72]. This supports the pilot evidence that, in response to EZN-2968, HIF-1alpha mRNA has the potential for inhibition as a cancer therapy target [65].
4.4.2. Inhibitors of HIF-1 Protein Translation
In normal conditions, proteasome enzymes degrade HIF-1alpha protein rapidly after its post-translational modification, but in hypoxic conditions, they stabilize by inhibiting post-translational modification enzymes before protein degradation [71]. Topotecan has been previously used for ovarian and lung cancer as second-line chemotherapy. Topotecan is a cytotoxic camptothecin analog that breaks double-stranded DNA during DNA replication. This, in turn, induces Top1-DNA cleavage complexes for maturation and poisons topoisomerase I [73]. Cardiac glycosides also affect the protein translation of HIF-1. In particular, digoxin is a powerful HIF-1 inhibitor. Digoxin inhibits the translation of HIF-1 using an mTOR-independent mechanism [65]. In advanced metastatic cancer patients, PX-478 is also a HIF-1 inhibitor in phase I clinical trials. In addition, reports have demonstrated antitumor activity and expression of HIF-1 in xenograft-tumour models [73].
4.5. Hypoxia-Inducible Factor-1 and Parasitic Infection
Hypoxia might be a physiological feature ranging from conditions like cancer to infection.
An oxygen-sensitive hydrolase enzyme mediates cellular hypoxia, which manages the protein stability of the hypoxic inducing transcriptional factor (HIF-1alpha). Once HIF-1alpha is stable, it binds its HIF-responsive elements (HRE) to the promoters of the TARGET gene to develop a broad transcriptional program. In response to a diversity of molecular infection and inflammation signals, HIF-1alpha also has a regulatory role in normalizing conditions. Pro-inflammatory cytokines, growth factors, and a wide range of infections can stimulate HIF-1alpha. It can induce the host's response to infection [74].
4.6. Sepsis and HIF-1 Alpha
HIF-1alpha could mediate a purposeful reprogramming of monocytes in sepsis. The plasticity of monocytes in human sepsis by HIF-1alpha may lead to protective actions such as antimicrobial activity, phagocytosis, and tissue restoration. This indicates the potential therapeutic use of HIF-1alpha as a human sepsis regulator [74]. Macrophages suffering from HIF-1alpha deficiencies have a deteriorated metabolic adjustment to hypoxia, resulting in reduced migration. In addition, HIF-1alpha-deficient macrophages possess a reduced ability to kill bacteria. Although the phagocytic rate in Group B Streptococcus was analogous, HIF-1alpha genetic modification impaired this bacterium through intracellular killing and lysis [75]. Other research using various pathogens has shown that hypoxia and HIF-1alpha have a potential role in controlling bacterial phagocytosis [76]. In intensive care facilities, sepsis is the main cause of death.
In this serious clinical condition, an immune response often injures the host with the lipopolysaccharide (LPS) acting as a strong immune cell activator via Toll-like receptor 4 (TLR4) by gram-negative bacteria. In vivo LPS-induced sepsis, LPS activates HIF-1alpha via a TLR4-dependent process and aids in the activation and lethality of cytokines.
HIF-1alpha is a decisive determinant of the sepsis phenotype through the production of inflammatory cytokines, including IL-l, IL-4, IL-6, IL-12, and TNF-alpha [77]. Inoculation in macrophages induced HIF-1alpha transcriptional activity in the macrophages of live or heat-inactivated gram-positive bacteria. These results show that HIF-1alpha myeloid deficiency decreases cell motility caused by gram-positive bacterial endotoxin and macrophage pro-inflammatory genes. This leads to fewer deaths and clinical symptoms of sepsis [78].
4.7. Bacterial Infection and HIF-1 Alpha
Bartonella henselae (bacillary angiomatosis disease) infections cause HIF-1alpha in vitro and In vivo activation, likely through metabolic alteration associated with hypoxia infection with the angiogenic bacterium. HIF-1alpha activation by human pathogenic Enterobacteriaceae of HIF-1alpha follows a common pattern of infections, such as iron defects in the host cells due to hypoxia-independent HIF-1alpha activation [79].
Shigella flexneri is a gram-negative bacterium that causes chronic and serious human bowel infections. The deterioration of the colonic epithelium allows the bacteria to access the liver and other organs. In a 24-hour laboratory study, S. flexneri invaded less cultivated hypoxia (6.5 percent O2) than normoxic conditions. The bacterium has been capable of invading hypoxia in rats. HIF-1alpha expression improved throughout the study in both hepatocytes, TNF-alpha secretion, and cell death. This bacterium was capable of invading rat hepatocytes while, due to this bacterium, hypoxia significantly decreased cell invasiveness [80]. Various host factors contribute to elevated HIF-1alpha expression and associated immune and metabolic responses during Mycobacterium tuberculosis (M. tb) infections. One factor is nitric oxide (NO), produced in infected murine macrophages by the action of inducible NO synthase (NOS2/iNOS). The induction can result in the redistribution of intracellular oxygen and the inhibition of PHDs (HIF-1alpha negative controls) by generating a positive feedback loop, leading to sustained high HIF-1alpha levels and an increase in macrophage activation.
During the infection of M. tb in granulomas in patients with pulmonary tuberculosis and macrophages (mouse and rabbit lungs), the development of NF-kB family members is controlled. In response to infection and inflammation with NF-kB activation, IkappaB kinases (IKK) are necessary for the phosphorylation-induced degradation of NF-B inhibitors. However, IKK-beta is important for HIF-1alpha aggregation in macrophages during bacterial infection. Shi, Y. H. and Fang, W. G [81]. then digest and translocate to the nucleus, allowing HIF-1alpha and other target genes to be transcribed [81].
In patients with colorectal malignancy or Crohn's disease (CD), multiple studies have reported the unusual closeness of intramucosal Escherichia coli or mucosa-related E. coli with obtrusive properties. Intrusive strains of E. coli (AIEC) colonize ileal CD sores and cause inflammation in the vicinity. In the irritated ileal epithelium of CD patients, pro-inflammatory cytokines (IL-1beta, IL-6, and IL-17) and reducing the articulation of mitigating cytokines, such as IL-10, stimulate HIF-1alpha expression. This inflammatory state facilitates the AIEC receptor. Using CEABAC 10 transgenic mice that express CEACAM6, AIEC microorganisms stimulate HIF-1alpha protein generation and VEGF/VEGFR flagging action. Downstream studies of human intestinal epithelial cells hushed for HIF-1alpha feature the basic role of ace angiogenic factors in this protein. This study presents the main role of AIEC microbes as an advertiser of provocative gastrointestinal tract problems and provides strong evidence that HIF-1alpha protein plays a primary role in this impact [82].
In another study, HIF-1alpha gene knockdown improved the AIEC survival rate in intestinal epithelial cells. The improved rate may be due to an autophagic flux impediment at autolysosomal maturation.
A novel HIF-1alpha feature seen in AIEC-linked xenophagy indicates that co-activation of HIF-1alpha expression and autophagy could be a potential novel treatment option to fight AIEC infection in CD patients [82]. Dramatically, Pseudomonas aeruginosa stifled the expression level of HIF-1alpha protein. Further interpretation showed that a subset of essential discharged ingredients associated with P. aeruginosa pathogenesis was subject to HIF-1alpha downregulation. The 2-alkyl-4-quinolone (AQ) majority detecting (QS) flagging atoms and particularly the Pseudomonas quinolone signal (PQS) are these components [83].
The discovery that P. aeruginosa AQ flagging atoms limited HIF-1alpha protein levels because bacterial segments tend to prompt the aggregation of HIF-1alpha protein. The key report of a bacterial factor equipped for this exploration was HIF-1alpha modification [83]. In the planned response of the aviation route cells to Staphylococcus aureus contamination, HIF-1alpha may play a role. Additionally, S. aureus-caused cells will not result in apoptosis. Trademark alarms for aureus disease include supporting consequent infection by gram-negative bacteria, such as P. aeruginosa, in patients with cystic fibrosis [84].
4.8. Viral Infection and HIF-1 Alpha
Protein from the Hepatitis C virus (HCV) induces HIF-1alpha by normoxic stabilization. The expression of HIF-managed genes and glycolytic enzyme coding was significantly upregulated. Similarly, the report indicated the expression of the HIF-managed genes in cell strains expressing sub-structural or nonstructural viral proteins with sub-genomic HCV debris. Stabilization and transcriptional activation of HIF-1alpha occurred in Huh7.5.1 cells containing life-derived infectious HCV cells and in liver biopsy samples from chronic hepatitis C patients. The HCV-related stabilization of HIF-1alpha became ineffective in anti-oxidant therapy [85]. A study by Nasimuzzaman, M., Waris, G., Mikolon, D., Stupack, D. G., and Siddiqui, A [86]. shows that HCV infection triggers the oxidant-mediated stabilization of HIF-1alpha and results in the expression of VEGF.
Additional research has demonstrated the relationship between the HCV core protein, HIF-1alpha induction, and VEGF expression when HCV and HIF-1alpha siRNA are transfected into Huh7.5.1 cells. These findings suggest that the acceptance of the articulation of HCV center quality in Huh7.5.1 cells was consequent upon HIF-1alpha expression.
In addition, the researchers of this recent study suggested that the long-term HCV protein articulation has created a sadness in mitochondrial oxidative phosphorylation. The HIF-1alpha action also triggers incitement to VEGF. The increased use of non-oxidative glucose pathways ensured cell endurance. This multi-faceted HCV-induced mitochondrial damage finally resulted in the control of HIF-1alpha modification and glycolytic compounds. Separate reports demonstrated equivalent findings in two frameworks of cell culture and HCV patients with hepatic biopsy [87].
A study of HCV infection revealed that HIF-1alpha stabilization by oxidants results in VEGF expression [86]. Infection of human fibroblasts with human cytomegalovirus (HCMV) stimulated the expression of HIF-1alpha. This effect was also stimulated by HCMV light (HCMV-U), suggesting that the reaction was produced without viral quality articulation by collaborating an infectious virion with the cell. Until 9 hours after contamination, the increase in HIF-1alpha protein was negligible. HCMV-U contamination of the explicit HIF-1alpha RNA demonstrates transcription stimulation [87].
Recent studies reveal the importance of HIF-1alpha in the reactivation of Kaposi's sarcoma-related herpesvirus (KSHV). The explicit interaction between the administrative factor 3 (vIRF3) of KSHV viral IFN and the HIF-1alpha subunit prompted HIF-1alpha modification and transcriptional enactment, which ultimately facilitated the development of endothelial tubes by initiating VEGF articulation. Under normal circumstances, the target area of vIRF3 was appropriate for HIF-1alpha officials to prevent their degradation.
This fact demonstrates that KSHV has produced a one-of-a-kind instrument to prove the stability of the HIF-1alpha protein by integrating into its genome a viral homologue of cell IRF efficiency, which can contribute to viral pathogenesis [87].
Human papillomaviruses (HPVs) are non-encompassed infections of DNA that assiduously contaminate stratified squamous epithelial keratinocytes. HPVs cause over 99 percent of cervical tumors [88]. In an examination, when HPV oncogenes were available, the levels of HIF-1alpha protein in hypoxia were elevated, which was true for a wide range of hazardous infections.
In addition, the increased HIF-1alpha expression showed an increase of some effectors downstream of the hypoxic reaction, indicating that HPV regulates a few parts of the hypoxic reaction of the cell [86]. HPV-16 E6 and E7 oncoproteins can accelerate the progression of non-small cell lung cancer by promoting tumor angiogenesis via the HIF-1alpha/VEGF pathways, which may provide a potential molecular target for HPV-related NSCLCC treatment [89].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19). The pathogenesis of COVID-19 starts when SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) on the surface of the ACE2 positive cells such as capillary endothelium and alveolar type II cells (AT2) [90, 91]. The virus infects these cells by inflammation and hypoxia, which triggers HIF-1alpha transcriptional activity [5]. During severe cases of COVID-19, HIF-1alpha activation can lead to cytokine storm by activation and stabilization of immune cells such as neutrophils and macrophages, leading to increased production of inflammatory cytokines by these cells, vascular leakage (by up-regulation of VEGF), and degradation of the alveolar-interstitial-endothelial complex barriers [92]. When HIF-1alpha activity is inhibited or its related signaling pathway is blocked, this can significantly reduce COVID-19 symptoms and patient death.
4.9. HIF-1alpha and Protozoan Parasitic Infection
Toxoplasma gondii is an obligatory pathogen of the intracellular protozoan. A few studies show that T. gondii infection elevated the gene expression level in cell reaction to hypoxia in T. gondii-infected cells. Hypoxia-inducible factor 1alpha limits the articulation of these genes. Toxoplasma contamination rapidly increased the number of HIF-1alpha units and the corresponding consistency articulation. In HIF-1alpha knockout cells, Toxoplasma development and resilience reduced to 3% oxygen. HIF-1alpha was not sufficient for parasite intrusion; however, it was required for parasite cell division and organelle conservation at 3 percent oxygen [93].
Various researchers have found that the measurement of transcripts encoding vascular endothelial growth factor (VEGF), glycolytic chemicals, glucose transporters, and transferrin receptors was upregulated by Toxoplasma contamination [94]. In an alternative study, hypoxia-instigated macrophages regulated Leishmania amazonensis (LA), an intracellular parasite that causes cutaneous metastatic sores [95]. Tumor Necrosis Factor-alpha, IL-10, IL-12, or nitric oxide, are not responsible for re-reducing hypoxia parasitism. Treatment with cancer prevention agents, N-acetylcysteine (NAC), is likely to be needed because the treatment retains a strategic distance from the leishmanicidal impacts of hypoxia on macrophages. Pollution from Leishmania amazonensis causes macrophage HIF-1alpha coordination [96]. In a different study by Kumar, V., Kumar, A., Das, S., Kumar, A., Abhishek, K., Verma, S., Mandal, A., Singh, R. K., Das, P [15]; Leishmania donovani infection induces a hypoxic environment in the host macrophages that triggers the expression of HIF-1alpha. Subsequently, the activated HIF-1alpha upregulates miR-210, which finally creates a favorable environment for the survival of L. donovani in the macrophages of the host by decreasing NF-kB-mediated pro-inflammatory immune responses [97, 98]. Araujo, A. P., Frezza, T. F., Allegretti, S. M., Giorgio, S [99]. showed that schistosomal granulomas are hypoxic, followed by the expression of HIF-1alpha and vascular endothelial growth factor (VEGF). The findings demonstrated a strong positive connection between the hypoxic tissue microenvironment created by S. mansoni infection and HIF-1alpha expression, which subsequently stimulates the development and growth of the granulomas.
CONCLUSION
The decline of oxygen content in the cellular environment induces HIFs, which have been implicated in the biology of cancer and infections. Hypoxia-inducible pathways have attracted great interest for treating inflammatory diseases and infections by viruses, protozoa or bacteria, among other pathogens. This review of the biochemical mechanisms of hypoxia signaling pathways provides a better understanding of how it could aid in the identification and development of new treatment strategies for parasitic diseases such as viral, bacterial, and fungal infections, as well as protozoa infections.
LIST OF ABBREVIATIONS
| HIFs | = Hypoxia-inducible Factors |
| VEGF | = Vascular Endothelial Growth Factor |
| TCA | = Tricarboxylic Acid Cycle |
| ROS | = Reactive Oxygen Species |
| HRE | = Hypoxia-responsive Elements |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The authors received no external funding for this work.
CONFLICTS OF INTEREST
The authors declared that there is no conflict of interest.
ACKNOWLEDGEMENTS
None.


