All published articles of this journal are available on ScienceDirect.
Astaxanthin's Impact on Colorectal Cancer: Examining Apoptosis, Antioxidant Enzymes, and Gene Expression
Abstract
Background
Colorectal cancer stands as the prevailing form of cancer affecting the digestive tract. Antioxidants have been observed to influence the activity of antioxidant enzymes and elevate the expression of genes within the apoptosis pathway. Consequently, this dynamic interplay appears to suppress the progression of colorectal cancer.
Objectives
This study aimed to evaluate the effect of astaxanthin on the expression of effective genes in apoptosis and the activity of antioxidant enzymes in colorectal cancer HCT-116 cells.
Methods
In this experimental investigation, HCT-116 cells underwent treatment with varying concentrations of astaxanthin for a duration of 24 hours. Subsequently, the expression levels of BAX, Bcl2, and caspase 3 genes were quantified using real-time PCR, while malondialdehyde levels and antioxidant enzyme activity were analyzed utilizing calorimetric methods.
Results
The analysis of gene expression outcomes revealed that astaxanthin elicited significant effects. It augmented the expression of BAX and caspase-3 genes, thereby promoting apoptosis while concurrently downregulating the expression of the Bcl2 gene. Consequently, this led to a decrease in malondialdehyde concentration, serving as an oxidative stress index. Additionally, the antioxidant activity of superoxide dismutase, catalase, and glutathione peroxidase showed significant increases in these treated cells.
Conclusion
Astaxanthin appears to modulate the antioxidant defense system within cancer cells. This is achieved by enhancing the activity of antioxidant enzymes while concurrently inhibiting cell growth and proliferation. Furthermore, the compound triggers apoptosis in HCT-116 cell lines, further contributing to its potential as a therapeutic agent in cancer treatment.
1. INTRODUCTION
Colon cancer is the most common cancer of the digestive tract. The disease is the second most common cause of death due to cancer in women after breast cancer, and in males, it is the third most common cause after lung and prostate cancer [1], which is why its primary prevention is particularly important. Lifestyle and eating habits seem to play an important role in the development of the disease; for example, low physical activity, low-fiber or high-fat diet, obesity, and the consumption of fast foods have provided favorable conditions for the disease [2]. Other risk factors for colorectal cancer include hereditary syndromes (autosomal dominant heredity), colon polyposis, non-polyposis syndromes (Lynch syndrome), inflammatory bowel disease, Streptococcus bovis infection, uret rostigmoidostomy, and tobacco [3]. Prospective studies have shown that the use of antioxidant-enriched nutrients has an inverse relationship with the risk of colorectal cancer [4, 5].
Oxidative stress plays an important role in the development and progress of cancer, so the use of exogenous antioxidants can be effective in preventing and treating this disorder [6]. Astaxanthin is a component of xanthophyll, which is one of the most well-known natural pigments [7]. It has been widely used in food and pharmaceutical formulations, as well as in cosmetic products. Small algae, such as H. pluvialis, Xantho- phyllomyces dendrorhous, and Phaffia rhodozyma, are natural producers of this compound. Astaxanthin is a fat-soluble compound that can accumulate in animal tissues, and so far, no side effects have been observed [8, 9].
Apoptosis, serving as a regulated process of cell death, plays a crucial role in eliminating undesirable or damaged cells. The impairment of apoptosis can disrupt the delicate balance between cell proliferation and cell death, leading to the potential development of cancer. Consequently, inducing apoptosis in pre-cancerous and cancerous cells represents a promising strategy for both cancer prevention and treatment [10]. The primary objective of this study was to assess the impact of astaxanthin on various aspects of human colorectal cancer HCT-116 cells. Specifically, the investigation aimed to evaluate the compound's potential to induce apoptosis, its influence on the expression of key genes involved in this process, and its effects on the activity of antioxidant enzymes.
2. MATERIALS AND METHODS
2.1. Cell Culture
The HCT-116 human colorectal cancer cell line was procured from the Pasteur Institute in Tehran, Iran. These cells were cultured and prepared for treatment in RPMI-1640 culture medium supplemented with 1% antibiotics (penicillin and streptomycin) and 10% FBS. The incubation process was carried out in a CO2 incubator at 37°C to maintain optimal conditions for cell growth.
2.2. MTT Assay
The MTT method was employed to ascertain the appropriate time and dosage of astaxanthin for treating the colorectal cells.
Using this method, different serial concentrations (5, 10, 25, 50, 100, 150, 200, 250, and 300 mM) of astaxanthin were added to the cells and incubated at 24, 48 and 72 hours. After some time, 20 μl of MTT was added to each well at a concentration of 5 mg/ml. After 4 hours of incubation in a dark place, the culture medium containing MTT was carefully extracted, and 200 μl DMSO was added to each well in a plate to dissolve purple formazan. After 15 minutes of incubation at room temperature, the absorbance of each well was read using an ELISA reader device at a wavelength of 570 nm (with a reference wavelength of 690 nm). Cellular survival IC50 (concentration that inhibits cell growth up to 50%) was reported based on the concentration curve (micromolar). Each of these experiments was repeated three times for better results, and cell survival was calculated according to the following formula:
% Cell viability = (OD treated cells / OD untreated cells) × 100
2.3. Astaxanthin Treatment
Based on the MTT results, the cells were subjected to treatment with astaxanthin at concentrations of 25, 50, 100, and 150 μM for a duration of 24 hours. Following this, RNA extraction and cDNA synthesis were carried out using the gene bioscience kit using the provided protocol. The RNA concentration was determined using the nanodrop device, and subsequently, one microgram of the extracted RNA was utilized for cDNA synthesis.Gene expression evaluations were performed using real-time PCR using the gene bioscience kit based on the relevant protocol according to 95˚C for 2 min as a polymerase activation step, 40 cycles of 95˚C for 15 sec for denaturation, and 60˚C for 40 seconds for primer annealing and elongation. Each run included a negative control (no cDNA). Table 1 shows the sequence of the forward and reverse primers.
Table 1.
| Gene Name | Sequences | Product Size |
|---|---|---|
| BCL2-F | TCGCCCTGTGGATGACTGA | 134 bp |
| BCL2-R | CAGAGACAGCCAGGAGAAATCA | |
| Bax-F | TGGCAGCTGACATGTTTTCTGAC | 195 bp |
| Bax-R | TCACCCAACCACCCTGGTCTT | |
| Cas3-F | TACCTGTGGCTGTGTATCCG | 134 bp |
| Cas3-R | TCAGTGTTCTCCATGGATACCT | |
| B2M-F | ACTGAATTCACCCCCACTGA | 167 bp |
| B2M-R | AAGCAAGCAAGCAGAATTTGGA |
Astaxanthin was purchased from Sigma Company with the following specifications: Sigma, Product Number A 9335, purity> 97%, and nm gy65rgtFirst. It was dissolved in DMSO and prepared with 20mM pbs. The amount of primary DMSO added to astaxanthin was reduced to less than 0.01 percent for each concentration.
2.4. Measuring the Activity of Antioxidant Enzymes
After the treatment of cells with varying concen- trations of astaxanthin and subsequent washing with PBS, 800 μl of lysing buffer was added to the cell samples. To facilitate further processing, the solutions were sonicated using an ultrasonic device, employing 2 to 3 cycles of 30 seconds each. Finally, centrifuges were carried out in 10000 g for 20 minutes at 4°C.
The resulting supernatant was preserved at -80°C until the enzyme activity was assessed.
2.5. Total Protein Concentration
The Bradford method was used to measure the total protein content in cell lysate samples.
A standard curve was generated by performing serial dilutions of BSA (Albumin).
2.6. Measuring Malondialdehyde
To measure malondialdehyde, 1500 μl of thiobarbituric acid (TBA) % 0.06 and 1000 μl of trichloroacetic acid (TCA) 1% were added to 100 μl of the samples, and the tubes were kept at 100 ° C for 30 minutes. After cooling, the sample was centrifuged for 15 minutes at 1000 rpm. The absorbance of the samples was then read at 535 nm in comparison with blank.
The amount of malondialdehyde in each sample was calculated in terms of nmol/mg protein [11].
2.7. Catalase Activity Assay
To measure the catalase, 1000 μl of 50 mM potassium phosphate buffer was poured in pH 7 and added to 50 μl of the cell sample. At the time of reading the absorbance of the samples, 50 μl of H2O2 was added, and the absorbance was recorded at zero, 30, and 60 seconds in 240 nm wavelength. The catalase activity was calculated in terms of unit /mg of protein [12].
2.8. Superoxide Dismutase Activity Assay
To measure the activity of superoxide dismutase, 725μl of Tris-EDTA buffer was added to 25μl of the sample, and at the time of reading absorbance, 80 μL of pyrogallol was added to the samples. The samples were recorded at 90 and 210 seconds in 420nm wavelength.
The activity of superoxide dismutase was quantified in units per milligram of protein [13].
2.9. Glutathione Peroxidase Activity Assay
For the measurement of the activity of glutathione peroxidase, the following steps were carried out:
200 μl of 0.4 molar Tris-Hcl buffer at pH=7, 100 μL of 1 mM sodium azide, 200 μl of 2 mM glutathione, and 100 μl of 0.2 mM H2O2 were added to 200 μl of the cell sample. The mixture was then incubated at 37°C for 10 minutes. Following the incubation, 0.4 ml of 10% TCA (Trichloroacetic acid) was added to the tubes, which were subsequently centrifuged for 3 minutes at 2000 rpm. Next, 25 μl of the supernatant was transferred into ELISA microplates.

The survival of HCT-116 cells was assessed at various concentrations of Astaxanthin after 24, 48, and 72 hours of treatment. However, none of the concentrations showed statistically significant effects on cell survival.
To the microplate wells, 140 μl of Tris-EDTA (pH = 8) and 30 μl of DTNB (5,5'-dithiobis (2-nitrobenzoic acid)) were added. The samples were then incubated at room temperature for 30 minutes. Finally, the absorbance of the samples was read at 420 nm. The glutathione peroxidase activity was determined in terms of units per milligram of protein [14].
3. RESULTS
The results of the MTT test are presented in Fig. (1). According to the results of the MTT method, concentrations of 25, 50, 100, and 150 μM of astaxanthin and 24h incubation time were selected for the treatment of HCT-116 cells (Fig. 1).
3.1. Gene Expression Results
3.1.1. Effect of Astaxanthin on Caspase Gene Expression
The result of the study on the HCT-116 cell line showed that although astaxanthin at all concentrations increased the expression of the caspase-3 gene compared to the control group, this increase was not statistically significant (P> 0.05) (Fig. 2).
3.1.2. Effect of Astaxanthin on the Expression of Bax Gene
Astaxanthin at all concentrations increased the expression of Bax gene expression compared to the control group, but this increase was not statistically significant (P> 0.05) (Fig. 3).
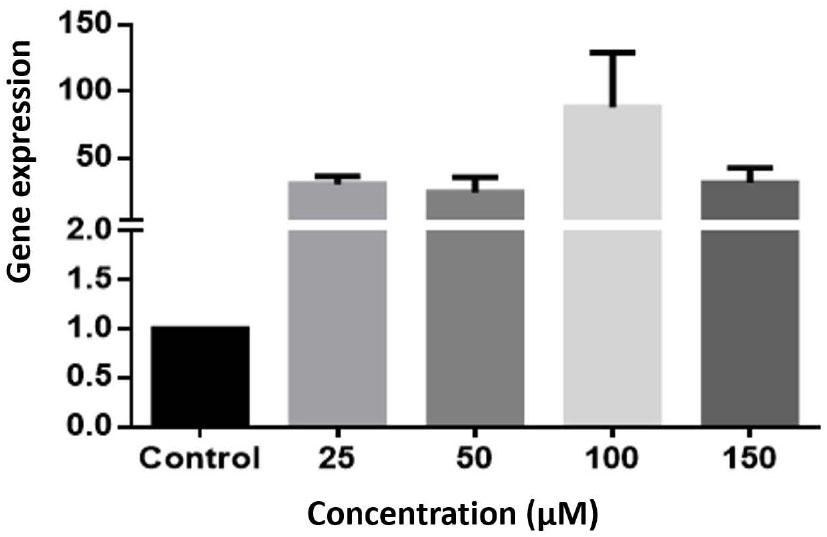
The expression of the Caspase 3 gene in the HCT-116 cell line was examined. The gene expression was analyzed using Real-Time PCR and compared to the control cells. The experiment was repeated three times, and the results were presented as Mean ± SEM (Standard Error of the Mean). However, none of the concentrations showed statistically significant differences compared to the control.
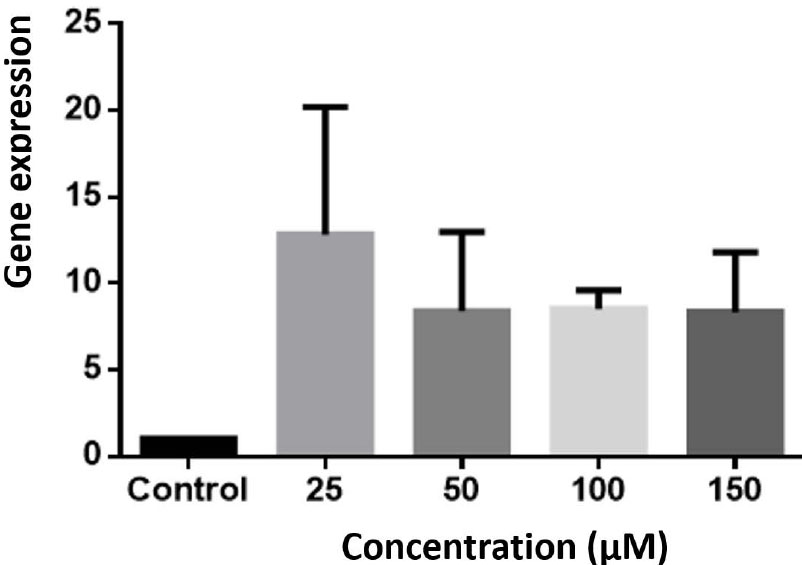
The study focused on the Bax gene expression in the HCT-116 cell line. The gene expression levels were assessed using the Real-Time PCR technique and compared to the control cells. The experiment was conducted three times, and the results were reported as Mean ± SEM (Standard Error of the Mean). However, none of the concentrations showed statistically significant differences compared to the control.

The investigation focused on the expression of the bcl-2 gene in the HCT-116 cell line. Real-Time PCR was employed to measure the gene expression levels, which were then compared to the control cells. The experiment was conducted three times, and the results were presented as Mean ± SEM (Standard Error of the Mean). However, none of the concentrations exhibited statistically significant differences compared to the control.
| MAD and Enzymes Activity |
Con 25 µM (mean±SD) |
Con 50 µM (mean±SD) |
Con 100 µM (mean±SD) |
Con 150 µM (mean±SD) |
Control 0 µM (mean±SD) |
Pvalue |
|---|---|---|---|---|---|---|
| MDA | 0.21±0.02 | 0.18±0.01 | 0.16±0.03 | 0.13±0.02 | 0.27±0.01 | Pvalue<0/05 |
| SOD | 23.1±4.2 | 25.3±3.1 | 26.8±2.6 | 29.4±2.1 | 18.02±1.9 | Pvalue<0/05 |
| GPX | 547.4±44.5 | 572.6±48.4 | 635.5±42.1 | 713.3±41.5 | 357.2±44.1 | Pvalue<0/05 |
| CAT | 1.74±0.4 | 1.87±0.1 | 2.07±0.6 | 2.18±0.3 | 1.12±0.11 | Pvalue<0/05 |
3.1.3. Effect of Astaxanthin on the Expression of bcl-2 Gene
According to the present study, astaxanthin at all concentrations reduced the expression of bcl-2 gene expression compared to the control group, but this decrease was not statistically significant (P> 0.05) (Fig. 4).
3.1.4. Effect of Astaxanthin on Malondialdehyde Levels and Antioxidant Enzyme Activity
The findings demonstrated a significant decrease in the mean malondialdehyde concentration across all astaxanthin concentrations when compared to the control group (P <0.05). However, there was no significant difference between the different concentrations of the treatment in reducing the mean malondialdehyde level (Table 2).
Experiments performed on HCT-116 showed that astaxanthin in all concentrations significantly increased the activity of superoxide dismutase compared to the control group (P <0.05). Furthermore, the comparison among different treatment concentrations revealed that the effect of the 150 μM concentration had a significantly higher impact on increasing the mean values of SOD compared to the other concentrations (Table 2).
According to the results of this study, only 150 μM astaxanthin concentration significantly increased the activity of the catalase (CAT) compared to the control group (P <0.05) (Table 2). All concentrations of astaxanthin significantly increased the mean glutathione peroxidase (GPX) level compared to the control group (P <0.05).
However, the effect of concentrations of 100 and 150 μM on average of GPX values was higher than that of other concentrations, which was statistically significant (Table 2).
4. DISCUSSION
Novel cancer treatment approaches focus on inducing apoptosis. Carotenoids were considered effective chemo-preventive agents in this approach [14]. Overall, reviewing existing studies confirms the role of carotenoids in decreasing the progression of cancer. In vitro studies have shown that astaxanthin has cytotoxic effects on cancer cells. Astaxanthin can affect cell activity by affecting various cellular processes and interacting with genetic mechanisms. Evidence suggests that induction of apoptosis is one of the most important mechanisms of carotenoids. The results of this study confirmed the pro-apoptotic effect of astaxanthin and the effectiveness of this antioxidant in HCT-116 cell cultures. Studies by Xiaojuan Liu et al. (2016) showed that the apoptosis of HCT-116 induced by astaxanthin was associated with an increase in the level of apoptosis-related proteins, such as caspase-3. In a study, the effects of astaxanthin on human breast cancer cells were studied. According to the results, astaxanthin, by increasing the expression of the caspase-3 gene in the MCF-7 cell line, leads to inhibition of growth and proliferation of cancer cells, which is similar to the present study on colorectal cancer [14, 15]. The investigation into the molecular mechanisms underlying astaxanthin's inhibitory effects on colorectal cancer cell growth revealed its influence on critical protein markers associated with cell proliferation, apoptosis, and the cell cycle. Immunological analysis displayed extensive alterations in signaling proteins due to astaxanthin treatment. The results demonstrated significant induction of p21Cip1/Waf1, P27, and p53 expression in HCT116 cells, accompanied by decreased expressions of CDK4, CDK6, and EGFR in the same cells. Additionally, astaxanthin led to the modulation of key proteins, including caspase-3, PARP, p-p38, p-JNK, and p-ERK1/2, contributing to its impact on cellular processes [16].
Thus, astaxanthin inhibits the growth and proliferation of colon cancer cells by regulating oncogenic signaling proteins, stopping the cell cycle, and inducing apoptosis.
Oxidative stress begins with the production of free radicals and reactive oxygen species. An imbalance in the production of ROS, or an impairment in antioxidant activity, leads to tumor progression [17]. Reducing the expression or decreasing the activity of antioxidant enzymes seems to be responsible for carcinogenesis [18]. Consequently, high regulation of the expression of antioxidant enzymes or enhancement of their activity is considered an effective way of preventing or treating cancer. For example, according to studies, excessive expression of the superoxide dismutase enzyme inhibits the growth of various types of breast cancer cells [19]. Many studies have also indicated the effect of carotenoids on the inhibition of cancer cells due to increased expression or activity of antioxidant enzymes. For example, lycopene was associated with an increase in the expression of antioxidant enzymes in the prostate cancer cell line [20]. Another study showed that antioxidants inhibited the growth of skin cancer cells by improving the activity of antioxidant enzymes and, as a result, by changing the antioxidant defense system [21]. In a study conducted by Li Zhang and Handong Wang in 2015 [22], it was revealed that astaxanthin effectively reduces intracellular O2 production in U937 cells by activating antioxidant superoxide dismutase and catalase enzymes [23]. These findings are consistent with the results obtained in our current study on colorectal cancer cells HCT-116.
The result of the study by Xiaobin Li et al. (2014) on type 2 diabetic rats showed that astaxanthin, by activating PI3K/Akt and decreasing the flow of oxidative stress pathway, led to a decrease in type 2 diabetes in rats and protected them against this disease. According to this study, astaxanthin significantly increased the level of GPx, GSH, and SOD and reduced MDA levels in the cortex and the hippocampus of diabetic rats, which is consistent with our studies on human cancer cells [24, 25]. Based on the results of this study, it seems that astaxanthin has been shown to increase its anti-proliferative activity by increasing the activity of antioxidant enzymes in the human colon cancer cell line.
CONCLUSION
The results of this study indicate the effect of astaxanthin on inhibiting the proliferation and induction of apoptosis in HCT-116 cells in colorectal cancer by increasing the expression of the genes that are effective in apoptosis and increasing the activity of antioxidant enzymes, although more studies are needed to confirm this theory.
AUTHOR’S CONTRIBUTION
Dr. Maryam Hormozi: conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
Designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript.
Dr. Parastoo Baharvand: Coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
LIST OF ABBREVIATIONS
| TCA | = Trichloroacetic acid |
| CAT | = Catalase |


