All published articles of this journal are available on ScienceDirect.
Amlodipine Protects against Methotrexate-Induced Acute Kidney Injury in Rats
Abstract
Background
Methotrexate (MTX) is a commonly used chemotherapy drug with known nephrotoxic effects, including the potential for acute kidney injury. However, the precise mechanism through which MTX induces nephrotoxicity remains unclear, though oxidative stress and direct toxic effects on renal tubules are believed to play key roles. Recent studies suggest that calcium channel blockers may offer promise in slowing down the progression of chronic kidney diseases.
Objective
The purpose of this study was to explore the potential of Amlodipine, a calcium channel blocker, to alleviate acute kidney injury caused by the administration of MTX in rats.
Methods
Three groups of twenty-four male Wistar rats were randomly assigned: Group 1—the control group was given normal saline orally. Group II, underwent five days of continuous administration of a single intraperitoneal (IP) dosage of 20 mg/kg MTX. The same dosage of MTX was given to Group III followed by an oral dose of Amlodipine at 5 mg/kg over the same period. Upon completion of the experiment, serum biochemical parameters, renal damage markers, oxidative stress, inflammatory markers, and kidney tissue histology were assessed.
Results
The results indicate that MTX administration significantly increased the levels of serum biochemical parameters, renal damage markers, inflammatory markers, oxidative stress markers, and induced alterations in kidney histology. However, the administration of Amlodipine following MTX treatment protected against these changes.
Conclusion
Amlodipine exhibits therapeutic potential in mitigating MTX-induced kidney injury in rats and its associated side effects.
1. INTRODUCTION
Acute kidney injury (AKI) is defined as the abrupt loss of excretory kidney function. It is a component of a group of illnesses known as acute kidney diseases and disorders (AKD), which are characterized by a progressive loss of kidney cells and nephrons along with a gradual decline in kidney function or chronic renal dysfunction [1, 2]. It affects not only critically ill patients but also non-critically ill individuals, necessitating prompt recognition by healthcare professionals [2]. Globally, the burden of AKI-related death is significantly greater than that of diabetes, heart failure, or breast cancer and mortality has remained high during the last 50 years [3]. AKI has long-term consequences, including a greater risk of cardiovascular events, progression to chronic kidney disease, and heightened long-term mortality [4]. Methotrexate is an FDA-approved folic acid antagonist and is highly effective in treating rheumatoid arthritis, juvenile idiopathic arthritis, and a range of cancers. It is also used in conditions like psoriasis, lupus, and inflammatory bowel disease [5, 6]. High-dose methotrexate (HD-MTX) at 500 mg/m2 or higher can be more effective during treatments but poses risks, including bone marrow suppression, lung and kidney problems, hematological issues, and infection risk. After HD-MTX, around 60% of patients experience reversible hepatitis, 25% develop hyperbilirubinemia, and lymphoma, and have a 9.1% chance of kidney problems [7]. Currently, available interventions used for treating high-dose methotrexate involve leucovorin and glucarpidase among others [8]. Following treatment, and within 15 minutes, glucarpidase might produce deoxy- aminopteroic acid by hydrolyzing MTX in circulation., resulting in a >95% reduction in plasma MTX levels. The application of glucarpidase in HDMTX therapy is subject to several limitations. It is prohibitively expensive, scarce, and has a brief half-life [9, 10]. Amlodipine (Amlo) is a calcium channel blocker specifically known for its improved vascular selectivity and prolonged action. It binds slowly and consistently to target receptors, ensuring a smooth onset of action and 24-hour blood pressure control [11]. Once-daily dosing enhances patient compliance and minimizes side effects. It is primarily used for treating hypertension and angina. However, recent research has uncovered its broader applications in antioxidant functions and apoptosis regulation [12]. Amlo is now employed in cerebrovascular stroke, neuro- degenerative diseases, leukemia, breast cancer, and more, either alone or in combination with other agents [13]. Studies conducted by Sinha and Agarwal showed that various categories of antihypertensive medications can be beneficial in managing hypertension in individuals with chronic kidney disease [14]. Therefore, this study examined amlodipine’s potential reno-protective effect in a rat model of acute kidney injury caused by methotrexate.
2. MATERIALS AND METHODS
2.1. Drugs and Chemicals
Amlodipine besylate 5mg strength capsules were purchased from Jamjoom Pharmaceuticals Co., Jeddah, Saudi Arabia with the Lot number (XM0221) while methotrexate (50mg/2ml) injections were purchased from Hospira Pharmaceutical Co., Illinois, United State with a Lot number (G024412AB).
2.2. Experimental
2.2.1. Animals
Twenty-four male Wistar rats, weighing between 170 g and 190 g were acquired from the animal facility of the Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia. For 7 days, the animals were made to acclimatize at a temperature between 24 °C and 26°C, relative humidity between 35 and 75 percent, and on a 12-hour light and dark cycle
2.2.2. Experimental Design
The rats were chosen at random and assigned to one of three groups, each containing eight animals with four animals per cage. Group I served as the control and received a 1ml/kg injection of normal saline. Group II received an intraperitoneal (IP) injection of MTX at a dosage of 20 mg/kg. Group III was administered an oral dose of 5 mg/kg Amlo (11) after receiving an IP injection of 20 mg/kg MTX. The treatments were administered over a period of five days. Following treatment, isoflurane was administered to rats as anesthesia and later euthanized. After the retro-orbital plexus collection of blood samples, the kidneys were removed, cleaned with PBS, and weighed. While the right kidneys were preserved in 10% buffered formalin for histopathological investigation, the left kidneys were kept at -80°C for tissue oxidative stress analysis. The revised Guide for the Care and Use of Laboratory Animals of The US National Institutes of Health served as the source of all recommendations for animal treatments, which were authorized by King Abdulaziz University's Faculty of Pharmacy's ethics committee, Jeddah, Saudi Arabia with approval number: PH-1442-75
2.2.3. Determination of Biochemical Parameters
After centrifuging the whole blood for ten minutes at 4˚C and 3000 rpm, serum samples were collected. The following parameters were measured using an ELISA kit from MyBioSource in California, USA: serum uric acid, urea, albumin, creatinine, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and neutrophil gelatinase-associated lipocalin (NGAL).
2.2.4. Assessment of Oxidative Stress and Antioxidant State in Kidney Tissue
One milliliter of 100 mM phosphate buffer (pH 7.4) containing one milligram of EDTA was used to homogenize 100 mg of frozen kidney tissue. The mixture was then centrifuged at 14,000 rpm for fifteen minutes at 4°C. Superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) levels were assessed in the supernatant using a commercial kit (MyBioSource, California, USA).
2.2.5. Histopathology
The kidney tissues were dehydrated using alcohol gradients, cleaned with xylene, and embedded in paraffin for 12 hours after being fixed in a 10% neutral-buffered formalin solution. Following a 5-µm thickness sectioning process using a microtome, paraffin-embedded blocks were fixed on slides for 60 minutes at 60°C in an oven. After deparaffinizing in xylene, the slides were rehydrated using alcohol gradients and washed for two minutes in distilled water. Hematoxylin and eosin solution were then used to stain the slides. Following that, the tissue sections were assessed using a light microscope, and images were captured for analysis and semi-quantitative scores assigned by a pathologist.
3. RESULTS
3.1. Effects of Amlo and MTX on Serum Biochemical Markers
First, the impact of amlodipine and MTX on the biochemical markers in the serum was investigated. As revealed in Fig. (1), the MTX-only administered group showed a noticeable elevation (p < 0.05) in serum urea, serum creatinine, serum uric, and albumin levels in contrast to the animals in the control group. However, rats treated with Amlo following MTX administration showed significant protection against changes in the serum biochemical markers (Fig. 1).
3.2. Effects of Amlo and MTX on Adhesion Molecules of Renal Injury Markers
Next, the effects of Amlo and MTX on adhesion molecules for renal injury markers in rats administered with MTX were quantified. Rats given MTX had significantly higher serum levels of ICAM-1, VCAM-1, and NGAL than the untreated animals in the control group (Fig. 2). These increases in the adhesion molecules were, nevertheless, noticeably protected against drastic changes following Amlo treatment (Fig. 2).
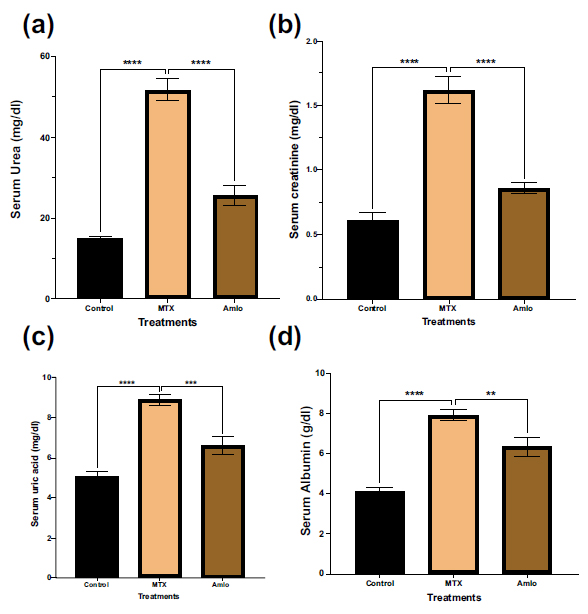
Effects of Amlo and MTX on serum biochemical markers. Serum urea (a), serum creatinine (b), serum uric acid (c), serum albumin (d). Results are displayed as mean ± SEM, (n =8). One-way ANOVA and Sidak's multiple comparisons were used to analyze the data. Compared to the MTX group, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
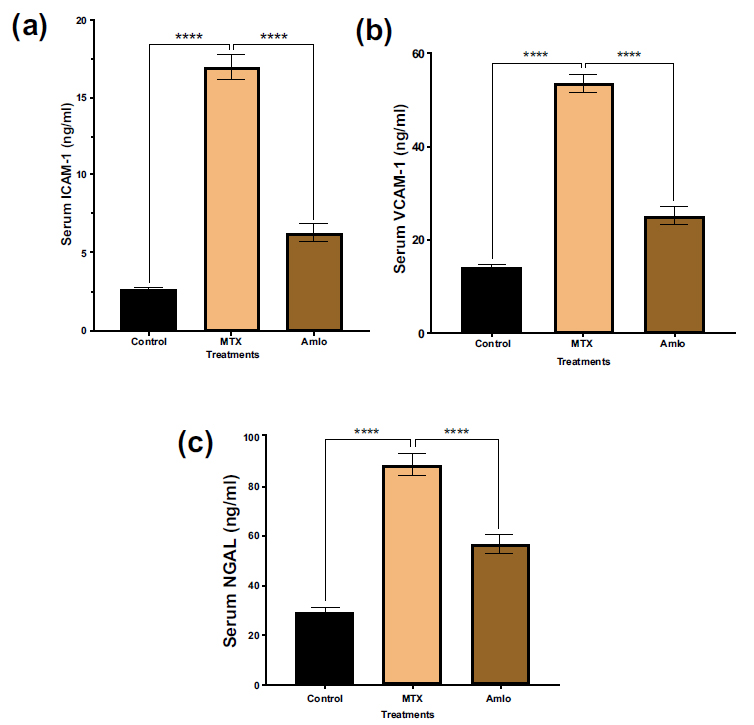
Effects of Amlo and MTX on adhesion molecules of kidney injury markers. Serum ICAM-1 (a), serum VCAM-1 (b) serum NGAL (c). Results are displayed as mean ± SEM, (n =8). One-way ANOVA and Sidak's multiple comparisons were used to analyze the data. Compared with the MTX group, ****P < 0.0001.
3.3. Effects of Amlo and MTX on Kidney Oxidative Stress Indicators
Next, the impact of Amlo and MTX on oxidative stress and antioxidant status indicators and oxidative stress were examined. As shown in Fig. (3), when compared to the animals in the control group, rats treated with MTX exhibited a substantial increase in tissue TBARS (p < 0.05). Furthermore, these rats showed a significant decrease in renal SOD and GSH (p < 0.05, Fig. 3) levels in comparison to animals in group 1 that received no treatment. In contrast, rats treated with Amlo after being administered with MTX showed a noticeable elevation in SOD and GSH (Fig. 3).
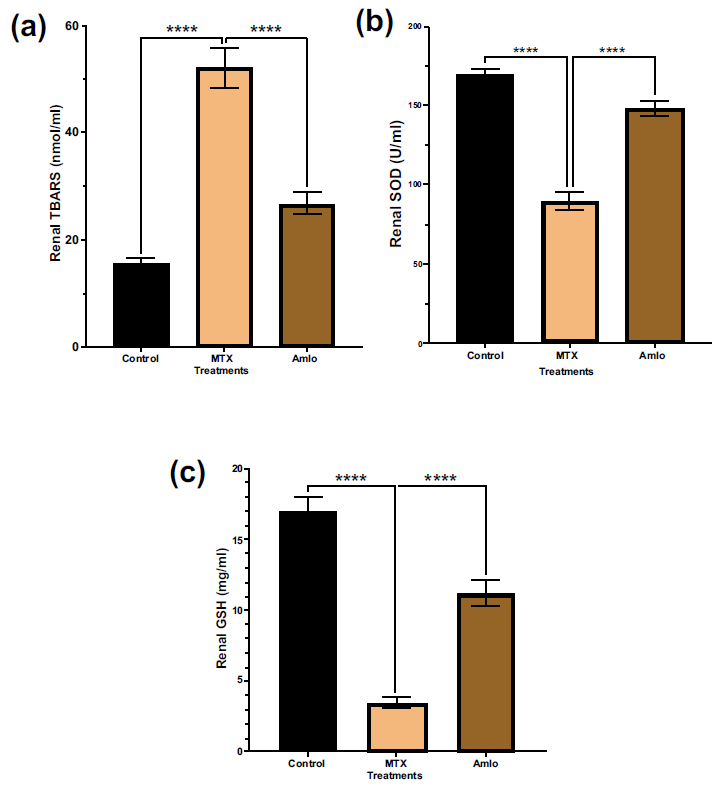
Effects of Amlo and MTX on kidney oxidative stress markers. Tissue TBARS (a), Tissue SOD (b), Tissue GSH (c). Results are presented as mean ± SEM, (n =8). One-way ANOVA and Sidak's multiple comparisons were used to analyze the data. Compared to the MTX group, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.4. Effects of Amlo and MTX on Serum Inflammatory Markers
Next, the impacts of Amlo and MTX on indicators of inflammation in serum samples of MTX-administered animals were examined. Animals treated with MTX had significantly higher serum levels of IL-6 and TNF-α compared to the control group (Fig. 4). In contrast, animals treated with Amlo after MTX treatment revealed significantly lower serum levels of IL-6 and TNF-α compared to rats treated only with MTX (Fig. 4).
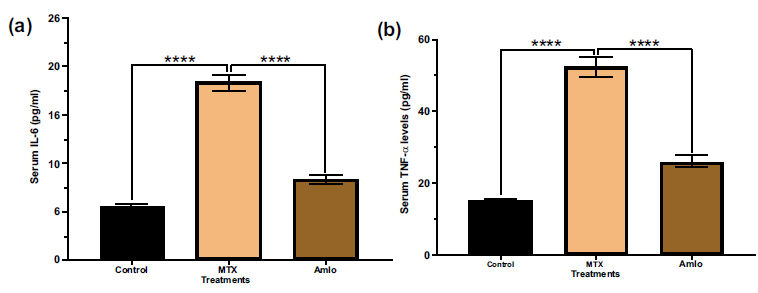
Effects of Amlo and MTX on serum inflammatory markers. Serum IL-6 (a), serum TNF-α (b) Results are presented as mean ± SEM, (n =8). Data was analyzed by One-way ANOVA followed by Sidak's multiple comparisons. Compared with the MTX group ****P < 0.0001.
| Histological Changes | Control | MTX | MTX + Amlo |
|---|---|---|---|
| Damaged renal corpuscle | 0 | 5 | 1 |
| Damaged glomerular capillaries | 0 | 5 | 1 |
| Damaged renal tubules | 0 | 5 | 1 |
| Cell aggregation | 0 | 5 | 1 |
| Dilated tubular lumen | 0 | 5 | 1 |
| Collapsed renal parenchyma | 0 | 5 | 1 |
Note: The histopathological ratings were given as follows: (o) normal; (1) mild; (5) severe.
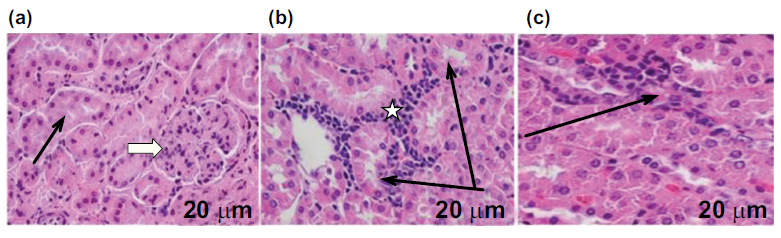
Effects of Amlo and MTX on kidney histological appearance. H&E staining from the kidney slice of animals in the control group reveals (a) typical histological architecture of the kidneys: renal corpuscles (white arrow), glomerular capillaries, and renal tubules (black arrow) (a), The kidney section of animals given MTX alone showed significant aggregates of infiltrating nonviable cells, smaller glomeruli and renal corpuscles, an enlarged tubular lumen, and a disorganized renal parenchyma with malformation and renal corpuscle atrophy (b), H&E staining of rat kidney sections after MTX and Amlo treatment reveals improved renal structure (c). Magnification: 400x; scale bar = 20 μm.
3.5. Effects of Amlo and MTX on Kidney Histological Appearance
Finally, the impacts of Amlo and MTX on kidney histological appearance were investigated. As demonstrated in Fig. (5), slices from the H &E-stained kidney of the untreated rats in the control group exhibited normal histological appearance, encompassing the renal tubules, renal corpuscle, and glomerular capillaries. In contrast, changes to kidney histological appearance produced by the injection of MTX in rats exhibited invasive viable cell aggregation. This is accompanied by a shrunken renal corpuscle and glomerulus; dilated tubular lumen, and collapsed renal parenchyma. On the other hand, Amlo-administered rats following an IP of MTX showed better kidney histological appearance (Table 1 and Fig. 5).
4. DISCUSSION
Previous studies have lent support to the role of amlodipine in drug-induced kidney disease in both human [15] and experimental animal models [16]. This study examined amlodipine's possible reno-protective properties against acute renal damage caused by methotrexate in rats. The findings demonstrated that rats given Amlo after MTX treatment revealed a significant drop in the serum levels of urea, creatinine, uric acid, and albumin, which had previously increased as a consequence of MTX administration. Furthermore, the finding revealed that Amlo protected rats against MTX-induced oxidative stress and inflammation, as well as structural alterations in kidney architecture.
Serum urea levels are crucial indicators for assessing renal function and detecting kidney diseases. Elevated urea levels signify renal dysfunction [17]. In this study, MTX administration caused a considerable elevation in the levels of serum urea, consistent with previous findings on MTX-induced kidney damage [18]. This increase was mitigated by Amlo administration, suggesting a potential renoprotective effect.
Creatinine, a byproduct resulting from the breakdown of creatine and creatine phosphate metabolism within muscle tissues, exhibits relatively consistent production levels proportional to muscle mass in individuals [19]. Assessment of serum creatinine levels serves as a diagnostic measure for kidney function impairment, with significant elevations indicating acute kidney injury [17]. In this study, rats administered with MTX showed a noticeable elevation in the levels of serum creatinine, consistent with findings reported by Hany et al. during their exploration of naringin's nephroprotective effects against MTX-induced renal toxicity [20]. This increase is attributed to MTX-induced delays in creatinine renal elimination, a phenomenon frequently associated with higher blood creatinine levels during MTX infusions. Treatment with Amlo following MTX administration demonstrated a decrease in serum creatinine levels, suggesting its role in mitigating MTX-induced renal impairment by attenuating serum creatinine elevation.
Uric acid, an end-product of purine metabolism [21], was found to be elevated in this study, and in concurrence with earlier findings by Alum et al. and Halil et al. [22, 23]. However, the increased uric acid levels induced by MTX were prevented with the administration of Amlo (Fig. 1c). MTX interferes with folate metabolism, suppressing purine and pyrimidine precursor synthesis. Studies indicate that MTX-induced alterations in de novo purine synthesis led to increased adenosine levels, which can be transformed into uric acid [5, 24]. This is supported by increased adenosine deaminase (ADA) activity previously observed in rats treated with MTX [25]. ADA plays a crucial role in purine metabolism, facilitating the conversion of adenosine to inosine, a precursor of uric acid. Strong ADA activity, indicative of rapid purine turnover and active salvage pathways [26], may contribute to the elevated serum uric acid levels induced by MTX treatment.
Albumin is a key serum protein that plays vital roles in maintaining colloidal osmotic pressure, binding various compounds, and providing plasma antioxidant activity. Decreased serum albumin levels are associated with reduced renal function [27]. With serum albumin, MTX exhibits approximately 50% binding capacity [28]. In this study, the MTX-only administered rats showed an increase in the levels of serum albumin which may be due to dehydration, possibly exacerbated by MTX-induced kidney damage [29]. This elevation was considerably decreased with Amlo treatment.
Intercellular adhesion molecule-1 (ICAM-1), found on endothelial cells and leukocytes, is linked to diabetic nephropathy as its levels increase with diabetes mellitus. Its overexpression is stimulated by inflammatory cytokines, with MTX-induced increase possibly due to an elevation in interleukin-1β and TNF-α [30]. This increase, observed in both this study and in a previous study by Gu et al. [31], was prevented with Amlo treatment.
In addition, Vascular Cell Adhesion Molecule-1 (VCAM-1), is a cell adhesion protein, that controls leukocyte migration and vascular adherence associated with inflammation in endothelial cells [32]. Its expression is triggered by pro-inflammatory cytokines like TNFα and ROS [32]. This finding aligns with Wang et al., who demonstrated VCAM-1 expression elevation after the administration of MTX to rats at a dose of 150mg/kg/day [33]. In contrast, in this study, Amlo administration prevented this elevation.
The lipocalin protein family's neutrophil gelatinase-associated lipocalin (NGAL) is a significant diagnostic for early AKI detection, increasing as early as 2 hours post-injury kidney [34]. In this study, MTX treatment elevated NGAL levels, which was similar to findings in prior studies [35]. This increase is attributed to MTX's inhibition of NGAL reabsorption in renal tubules [36]. However, Amlo administration reduced MTX-induced NGAL elevation, suggesting enhanced NGAL reabsorption by the kidneys.
Thiobarbituric acid reactive substances (TBARS) are byproducts of lipid peroxidation and serve as a standard marker for oxidative stress-induced lipid peroxidation [37]. MTX induces ROS production, resulting in lipid per- oxidation and mitochondrial dysfunction [38]. This study showed increased TBARS levels in the MTX-administered group, which aligns with a previous study by Dalaklioglu et al. [39]. Amlo, on the other hand, significantly protected against increased TBARS levels, suggesting its protective effects against lipid peroxidation. Previous studies have shown that MTX inhibits malic and cytosolic NADP-dependent dehydrogenases, which may decrease NADPH availability and impede nucleic acid metabolism [40]. The antioxidant defense mechanism is compromised by the MTX-induced decline in GSH levels, thus rendering cells more susceptible to ROS [40]. Previous studies confirm MTX-induced GSH depletion, which was protected by Amlo administration. The antioxidant enzyme Superoxide dismutase (SOD) is crucial for defense against ROS [41]. MTX decreases SOD levels, consistent with the study of Erdogan and colleagues [42], while Amlo administration protected against this decrease, possibly by scavenging excess free radicals and enhancing antioxidant status.
Interleukin-6 (IL-6) is a cytokine that is well-known for its multifaceted functions in inflammation, hematopoiesis, and immune responses. However, sustained elevation of IL-6 production may lead to immune system disorders [43]. Methotrexate has been shown to increase IL-6 production, possibly by reducing serum IL-6 clearance [44]. Consistent with a prior study by Abdel-Daim et al. [45], the results of this study showed elevated serum IL-6 levels in the MTX-administered group, which were protected by treatment with Amlo.
Tumor necrosis factor alpha (TNFα) is a pro-inflammatory cytokine implicated in activating inflam- matory molecules, cell adhesion proteins like VCAM-1 and ICAM-1 as well as cytokines [46]. Elevated TNFα levels have been linked to advanced malignant growth and inflammation. Chronic inflammatory conditions such as diabetes and hypertension often exhibit increased TNFα activity, impacting transporter function, the transport of nephrons, and renal hemodynamics. TNFα also promotes organ damage, cell death, and immune cell infiltration [47]. The observed elevation in TNFα levels in rats administered with only MTX may result from macrophage infiltration into kidney cells, as supported by a previous study [48]. Treatment with Amlo protected against the elevation in the levels of TNF-α caused by the administration of MTX, suggesting one potential mechanism by which Amlo exerts its renal protective effects.
In this study, invasive cell aggregation, glomerulus, and renal corpuscle decrease, tubular lumen dilatation, and renal parenchyma collapse were observed in the histological sections of MTX-only administered rats. These findings are in line with those of the [23, 49] studies which demonstrated comparable impacts of MTX on renal tissues. In addition, there was a remarkable improvement in the histological architecture of the kidneys of rats treated with MTX and Amlo.
CONCLUSION
This study suggests that MTX administration can lead to kidney damage, inflammation, and oxidative stress, although these effects can be greatly avoided by subsequent treatment with Amlo. This underscores the potential therapeutic effect of Amlo in protecting against MTX-induced kidney impairment and associated complications. However, further study is required to completely elucidate the mechanism at play and to establish the appropriate dose and timing of Amlo administration following MTX treatment.
LIST OF ABBREVIATIONS
| Amlo | = Amlodipine |
| AKI | = Acute kidney injury |
| MTX | = Methotrexate |
| HDMTX | = High-dose methotrexate |
| ADA | = Adenosine deaminase |
| ICAM-1 | = Intercellular adhesion molecule-1 |
| VCAM-1 | = Vascular Cell Adhesion Molecule-1 |
| SOD | = Superoxide dismutase |
| GSH | = Reduced glutathione |
| TBARS | = Thiobarbituric acid reactive substances |
| IL-6 | = Interleukin-6 |
| TNFα | = Tumor necrosis factor-alpha |
| NGAL | = Neutrophil gelatinase-associated lipocalin |
| ROS | = Reactive oxygen species |
ETHICAL STATEMENT
This study was approved by the ethics committee of the Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia (reference number: PH-1442-75). All the reported experiments were in accordance with The US National Research Council's “Guide for the Care and Use of Laboratory Animals”.


