All published articles of this journal are available on ScienceDirect.
Long-term Hypoxia Inhibits Sphere Formation on PC-3 and MDA-MB-231 Cell Line Models
Abstract
Background
Cancer stem cells (CSCs) represent a relatively small subset of cells within tumors, capable of self-renewal and associated with metastasis and cancer recurrence. While conventional chemotherapy targets actively dividing bulk tumor cells, dormant CSCs remain unaffected and survive. Hypoxia or deprivation of oxygen supply is a common feature of solid tumors, which plays a critical role in metastatic progression and CSC maintenance. However, the cellular responses to hypoxia might be influenced by many factors, including the severity, duration, and other specific characteristics of this stress.
Objective
In our study, we assessed the impact of long-term hypoxia on the CSCs population in 5 cell lines representing 5 different tumor types.
Methods
We assessed and characterized the effect of oxygen concentration on CSC population using the sphere formation assay. The protein levels in tumor spheres were examined by western blot analysis.
Results
Long-term hypoxia inhibited sphere formation by PC-3 and MDA-MB-231 CSCs. Moreover, chronic hypoxic stress suppressed cell proliferation in tumor spheres in all 5 tested cell lines: SNB-19, HCT116, MDA-MB-231, NCI-H460 and PC-3. This effect was accompanied by PCNA downregulation in tumorspheres derived from NCI-H460 and PC-3 cells.
Conclusion
The prolonged hypoxic conditions impede tumor sphere formation by PC-3 prostate CSCs, primarily through the downregulation of PCNA levels. The specific cellular response to hypoxia depends on the duration and, supposedly, other specific features of this stress.
1. INTRODUCTION
Cancer stem cells (CSCs) have been characterized as cells within a tumor that possess the ability to self-renew and generate the heterogeneous lineages of cancer cells comprising the tumor [1]. In simpler terms, CSCs are a subset of cells capable of initiating new tumors and typically represent a small proportion of the overall cancer cell population within a tumor [1]. The proportion of CSCs within a tumor can vary widely, ranging from 0.02% to 25%, with higher ratios often observed in undifferentiated tumors such as leukemias and lymphomas, and lower percentages more commonly found in solid tumors [2]. However, it has been suggested that not all cancers follow the classical CSC model [3]. Instead, according to the CSC plasticity model, both CSCs and non-CSCs can dynamically switch between two phenotypic states [4], exhibiting varying levels of tumor-initiating capacity, treatment response, clonogenic ability, and expansion potential [2]. CSCs can be identified and isolated based on cell surface markers such as CD44 and CD133, functional markers like aldehyde dehydrogenase activity, and most importantly, their tumor-initiating capacity [5-7].
The sphere formation assay is a commonly employed method to assess and characterize the CSC population residing in tumors or cancer cell lines. This assay relies on the ability of CSCs to proliferate and form tumor spheres in non-adherent culture conditions using serum-free media [8].
Accumulating evidence suggests that CSCs play a pivotal role in the uncontrolled growth of malignant tumors, therapy resistance, cancer recurrence, develop- ment of protective niches, evasion of the antitumor immune response, and tumor metastasis [9-11]. Mito- chondrial biogenesis is crucial for maintaining the CSC phenotype [12]. During the transition from anchorage-dependent to anchorage-independent growth, CSCs derived from breast cancer cell lines undergo a metabolic shift from glycolysis to oxidative mitochondrial phenotype [13]. At the same time, under hypoxic conditions, breast CSCs redirect their metabolism from oxidative phosphorylation to the glycolytic pathway [14]. The biological activities of CSCs are regulated by several pluripotency transcription factors: homeobox transcription factor Nanog (Nanog), octamer-binding transcription factor 4 (OCT4), sex-determining region Y-box 2 (Sox2) and Krüppel-like factor 4 (KLF4) [15]. The same factors specifically contribute to the reprogramming of somatic cells, inducing a state similar to that of stem cells [16]. These four master transcription factors have been recognized as pivotal components of the gene regulatory network responsible for coordinating the maintenance of pluripotency and differentiation in stem cells [17, 18]. Oct4 and Nanog are significantly overexpressed in non-small cell lung carcinoma [19], breast carcinoma tissues and the MCF7 breast cancer cell line [20]. Moreover, in prostate tumors, the expression of these 2 transcription factors correlates with HIF1α-positive regions and prostate tumor Gleason score [21].
Hypoxia refers to a condition where a particular region of the body or the entire body is deprived of sufficient oxygen supply at the tissue level, resulting in impaired normal physiological functions and homeostasis [22]. Thus, maintaining oxygen homeostasis is vital for cell growth and survival [23]. Physiological oxygen levels in healthy tissues typically range from 4.6% to 9.4% O2, varying depending on the specific organ [24]. In contrast, oxygen concentrations within tumors are generally lower, averaging around 1-2% O2 or even less [25]. Deregulation of cellular energetics is a hallmark property of cancer cells [26]. Hypoxia is a common feature of most solid tumors and is known to promote malignant progression, metastatic dissemination, chemotherapy resistance, and ultimately, poor patient prognosis [27].
Hypoxia-inducible factors (HIFs) play a crucial role in controlling the metabolic, functional, and vascular adaptations to low oxygen tension in healthy and tumor cells. HIFs regulate a variety of target genes involved in angiogenesis, proliferation, apoptosis, survival, and adaptation to low oxygen levels [28]. Elevated expression levels of HIF1α or HIF2α in tumor biopsies from breast, pancreatic, or lung cancer patients have been associated with increased rates of metastasis and mortality [29]. The expression of NANOG is regulated by the HIF1 [30]. Moreover, HIF leads to CSC enrichment within hypoxic tumors by recruiting NANOG as a coactivator for telomerase reverse transcriptase (TERT) gene transcription. The TERT gene encodes the telomerase reverse transcriptase that maintains telomere length and is essential for the process of stem cell self-renewal [31].
Hypoxia can manifest in different forms, ranging from mild to severe. Acute and chronic hypoxia are the two most common and well-described subtypes of tumor hypoxia [32]. Acute hypoxia arises from perfusion disturbances in small blood vessels, resulting from irregular erythrocyte flow or local uncontrolled cell mass increase. It typically lasts from minutes to hours [33]. In contrast, chronic hypoxia is caused by limitations in oxygen diffusion due to the overproliferation of cancer cells and increased distance to the nearest blood vessels. Under chronic hypoxia, cells experience low oxygen tensions for extended periods, often exceeding 24 hours, which can lead to cell death if reoxygenation does not occur [33]. The third type of hypoxia is cyclic hypoxia, characterized by transient periods of hypoxia due to the presence of immature vasculature. In response to hypoxia, cancer cells release angiogenic factors that promote neovascularization. However, even after the formation of new blood vessels, hypoxia persists in the core areas of solid tumors [34]. Oxygen concentration gradually decreases from the periphery to the center of the tumor sphere, with the center becoming completely anoxic or oxygen-deprived for tumor spheres larger than approximately 170 µm in radius, as estimated in lung cancer tumors by Thomlinson and Gray [35]. This diffusion-limited or chronic hypoxia is commonly observed in solid tumors.
The different subtypes of hypoxia described above can elicit distinct stress-related responses within the tumor, thereby influencing tumor development and treatment response [32]. Hence, it is crucial to consider the type and duration of hypoxia exposure when assessing the impact of hypoxic stress on cancer cells and its specific biological consequences. In this study, we examined the effects of long-term hypoxia on tumorsphere formation by CSCs. We utilized five different cell lines representing five cancer types known for their propensity to develop metastases: MDA-MB-231 (breast cancer), SNB-19 (glioblastoma), NCI-H460 (lung cancer), PC-3 (prostate cancer), and HCT116 (colon cancer). It is important to note that breast, lung, colon and prostate cancers are the most frequently diagnosed cancers according to worldwide statistics [36, 37]. We included the SNB-19 cell line in this study because hypoxia is a well-known common feature of glioblastoma tumors [38]. Also, HIF-1 was reported to be an important driver of tumor progression [39]. Finally, the above-mentioned cell lines were chosen based on their ability to form tumor spheres in serum-free suspension conditions. Furthermore, we assessed the impact of hypoxia on the expression of proliferating cell nuclear antigen (PCNA), a marker of proliferation, and NANOG, a marker of stem cells, in tumorspheres. The goal of this study was to assess the effect of prolonged hypoxic and nutrient-deprived stress on CSC features across 5 different cancer types. Here, we wanted to expand our knowledge of how the long-term hypoxia impacts the cellular fates during cancer progression. The obtained results complement published data and highlight the importance of exposure duration on CSC’s stress response.
2. MATERIALS AND METHODS
2.1. Cell Culture
The PC-3 prostate cancer epithelial cell line (ATCC number: CRL-1435), triple-negative breast cancer (TNBC), MDA-MB-231 cell line (ATCC number: HTB-26), glio- blastoma cell line SNB-19 (ATCC number: CRL-2219), colon cancer cell line HCT116 (ATCC number: CCL-247), and lung cancer cell line NCI-H460 (ATCC number: HTB-177) were generously provided by Dr. Jenny L. Persson's laboratory at Umea University, Sweden. MDA-MB-231, SNB-19, HCT116, and PC-3 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM), while NCI-H460 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium, both obtained from PanEco, Russia. The culture media were supplemented with 10% fetal bovine serum (Gibco Life Technologies, USA) and 10% penicillin-streptomycin (PanEco, Russia). Cells were maintained in a 5% CO2 incubator (Eppendorf, Germany) at 37 °C. Upon reaching 80% confluency, the cells were detached using 0.25% trypsin (Gibco Life Technologies, USA) and subsequently subcultured at a 1:3 ratio in six-well plates (SPL Life Sciences, South Korea). For further experiments, cells at the logarithmic growth phase, when they reached 70-80% confluency, were selected.
2.2. Sphere Formation Assay
To prepare the sphere culture medium (SCM), B27 supplement (final concentration 1x, PanEco, Russia), EGF, and FGF2 (SCI store, Russia) were added to a 1:1 mixture of DMEM and F12 (PanEco, Russia), with a final concentration of 40 ng/ml for EGF and FGF2. Cells at the subconfluent level were trypsinized, and the resulting cell pellets were resuspended in SCM at an approximate concentration of 10^6 cells/ml. Subsequently, 100 µl of the cell suspension was transferred into a tube containing 900 µl of SCM. The cells were gently pipetted multiple times using a 1.0 ml sterile insulin syringe needle to disintegrate cell clumps. The cell number was determined using a Bürker counting chamber (Thermo Fisher Scientific, USA). For seeding, 500 cells/dish (HCT116) or 2000 cells/dish (MDA-MB-231, NCI-H460, PC-3, SNB-19) were plated in non-adherent 35x10 mm culture dishes (SPL Life Sciences, South Korea) containing SCM. The cells were cultured for two weeks in a humidified incubator at 5% carbon dioxide and either 21% (normoxic) or 2% (hypoxic) oxygen conditions. Hypoxic conditions were achieved using a custom-made BACTROX hypoxic chamber (Shel Lab, USA). The spheres were counted, and photomicrographs were captured using a Leica DM IL Led Fluo microscope (Leica Microsystems, Germany) equipped with a Leica DFC365 FX camera. The diameter of the tumor spheres was measured using Leica Application Suite X (LAS X) software (Leica Microsystems, Germany). All experiments were conducted in technical duplicates with three independent experiments.
2.3. Western Blot Analysis
The spheres were lysed in Radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor (PI, Thermo Fisher Scientific, USA) and phenylmethane- sulfonyl fluoride (PMSF, Thermo Fisher Scientific, USA) for 40 minutes on ice, followed by centrifugation. Protein concentrations in the lysates were determined using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, USA). The proteins were separated based on molecular weight using polyacrylamide gel electrophoresis with 4% stacking gels and 12% separating gels. A total of 30 µg of protein sample mixed with 4x loading buffer was loaded into each well of the gel. After electrophoresis, the proteins were electroblotted from the gel to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories, USA) using a semi-dry transfer method following the standard protocol provided by Bio-Rad. The accuracy of the transfer was verified by staining the gel with Ponceau S (Sigma-Aldrich, USA). Non-specific binding was blocked by incubating the membrane overnight in a 5% dry milk solution in tris-buffered saline with Tween-20 (TBST) buffer. Subsequently, the membranes were incubated with primary antibodies diluted at a ratio of 1:200, including PCNA (36 kDa; cat. no. sc-7909, Santa Cruz Biotechnology, USA) [12, 40], NANOG (46 kDa; cat. no. sc-134218, Santa Cruz Biotechnology, USA) [41] and β-actin (42 kDa; cat. no. A00730, Genscript Biotech, USA) [42]. Horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit IgG cat. no. A16110 or anti-mouse IgG cat. no. A16078; Thermo Fisher Scientific, USA) were then applied to the membranes. The visualization of the labeled proteins of interest was achieved by incubating the membranes in a Bio-Rad HRP-substrate solution for several minutes.
2.4. Statistical Analysis
The in vitro data are presented as the mean ± standard error of the mean (SEM), obtained from at least three independent experiments, with a minimum of two technical replicates per experiment, unless otherwise stated, a p-value of ≤0.05 was considered significant. Statistical significance was measured using the Student's t-test.
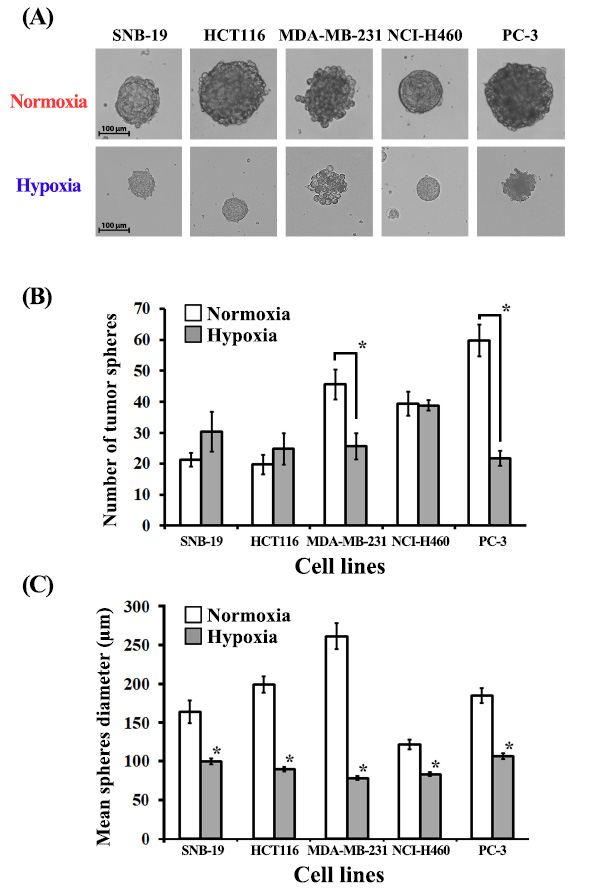
3. RESULTS
3.1. Long-term Hypoxia Inhibits Sphere Formation by PC-3 and MDA-MB-231 Cancer Stem Cells
The tumor-sphere formation assay is a commonly employed method to assess the frequency of tumor-initiating cells within a heterogeneous population of cancer cells [43]. Tumor spheres, dense and spherical structures, arise from the proliferation of a single cancer stem cell (CSC) and are cultivated under serum-free suspension conditions [44]. This culture environment favors the survival and proliferation of CSCs or progenitor cells, which possess the ability to generate new spheres. This characteristic is a shared property among stem cells and tumor-initiating cells from various lineages. In suspension culture, CSCs can form tumor spheres within 10-14 days, with diameters ranging from 50 to 250 µm (Fig. 1A). The total number of tumor spheres generated is commonly used as an indicator of the CSC/progenitor cell proportion within the cancer cell population in vitro [45]. Notably, compared to other cell lines, MDA-MB-231 exhibited a lower capacity to form well-rounded and compact tumor spheres under both normoxic and hypoxic conditions (Fig. 1A). Interestingly, under hypoxic conditions (2% O2), the total number of formed spheres was significantly reduced in two of the tested cell lines: PC-3 (representing prostate cancer) and MDA-MB-231 (representing triple-negative breast cancer) (Fig. 1B). However, for the remaining three tested cell lines (SNB-19, HCT-116, NCI-H460), no significant differences in sphere-forming efficiency were observed between normoxia and hypoxia. It is worth noting that with SNB-19 cells, there was a trend towards increased total neurosphere formation under hypoxic conditions (30 spheres in hypoxia compared to 21 in normoxia).
3.2. Long-term Hypoxia Inhibits Cell Proliferation in Tumor Spheres Developed by SNB-19, MDA-MB-231, PC-3, HCT116, NCI-H460 Cancer Stem Cells
A notable distinction in the mean diameter of tumor spheres was observed between normoxic and hypoxic conditions for all cell lines examined (Fig. 1C). Specifically, the average sphere size was 186 µm under normoxia, whereas it decreased to 91 µm under hypoxia. The induction of hypoxic stress exerted a significant influence on the behavior of CSCs cultivated in suspension, thereby impacting sphere formation. We propose that continuous exposure to hypoxic conditions leads to a deceleration of cell division rates within the tumor spheres. Consequently, the persistence of hypoxia gives rise to discernible variations in sphere size contingent on the availability of oxygen.
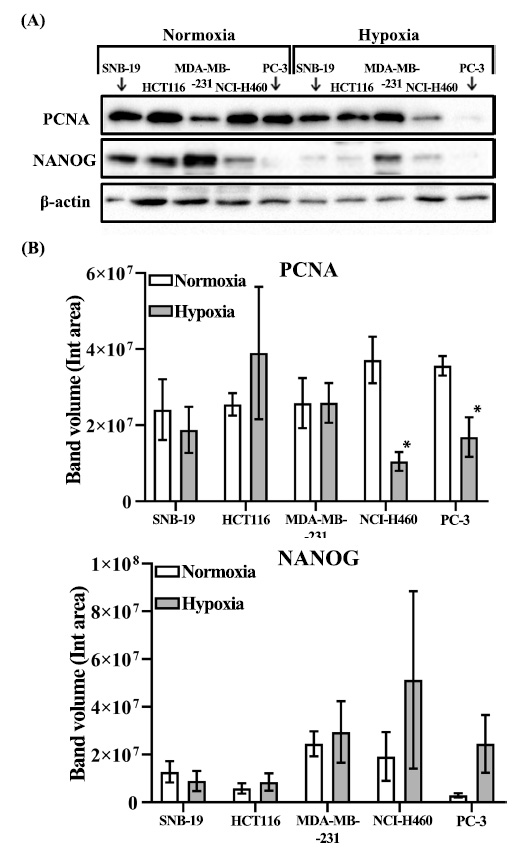
3.3. Long-term Hypoxia Leads to PCNA Downregulation in PC-3 and NCI-H460 Tumorspheres
Considering the observed reduction in the average diameter of spheres under hypoxic conditions across all tested cell lines, we proceeded to investigate the expression of commonly employed markers associated with proliferation and self-renewal. Ki-67 and PCNA are widely recognized indicators of tumor cell proliferation, with PCNA being a crucial component involved in nucleic acid metabolism and the replication and repair processes [46]. In this study, we investigated the expression levels of PCNA and NANOG in tumorspheres derived from five different cell lines, under both normoxic and hypoxic conditions (Fig. 2A, B). Notably, our findings revealed a substantial reduction in PCNA protein levels in PC-3 and NCI-H460 tumor spheres under hypoxic conditions (Fig. 2B). Conversely, the levels of NANOG remained unaffected by the hypoxic environment across all five tested cell lines (Fig. 2B).
4. DISCUSSION
Metastasis is recognized as the leading cause of cancer-related morbidity and mortality, accounting for approximately 90% of cancer-related deaths [47]. Advances in early detection and novel treatment strategies have significantly improved cancer survival rates. However, conventional radiotherapy and chemotherapy primarily target rapidly dividing bulk tumor cells, leaving behind a reservoir of treatment-resistant CSCs that can lead to fatal relapses years or even decades later [48].
In 2010, Li et al. provided direct evidence of CSCs contributing to metastasis. Their study demonstrated that breast CSCs, identified by the stem cell markers CD44+ and CD24-/low, initiated primary tumor formation and subsequently developed lung metastases [49]. Metastasis occurs due to a combination of factors such as oxygen and nutrient deprivation, lactic acid production, and immune responses [50]. Hypoxia, in particular, has been implicated as a crucial environmental factor promoting metastatic progression in various tumor types [25, 51, 52]. Upregulation of hypoxia-inducible factors (HIF-1 and HIF-2) has been associated with increased distant metastasis and poor overall survival in many cancers. Immunohistochemical analysis of primary tumor samples has revealed a correlation between HIF-1 expression and metastasis in patients with lung [53], esophageal [54], breast [55], and pancreatic cancers [56]. In melanoma, both HIF1α and HIF2α independently facilitate metastasis by regulating cell invasion and remodeling the extracellular matrix [57].
Our study aimed to investigate the impact of oxygen availability on CSCs. We employed the sphere formation assay, a three-dimensional culture technique that serves as a functional test for stem/progenitor cell activity in vitro. Among the five tested cell lines, we observed a significant reduction in the total number of spheres under hypoxic conditions in PC-3 and MDA-MB-231 cells. In tumorspheres derived from PC-3 cells, this was accompanied by PCNA downregulation. Similarly, a significant decrease in relative mRNA expression of PCNA in hypoxia was detected before in HepG2-derived tumorspheres [58]. In particular, hypoxic HepG2 spheres exhibited higher proliferative capacity on the 7th day of culture with both higher cell yield and PCNA marker expression; but on the 15th day the obtained results were completely opposite: hypoxia significantly reduced their cell yield along with downregulation of PCNA expression. Thus, authors suggest that a hypoxic microenvironment promotes tumor sphere growth during the initial phase, but in the later stage of cancer progression prolonged hypoxia causes alterations in their cellular features. In our study, we did not detect any significant differences in tumorsphere count with the other 3 tested cell lines. We believe this can be related to the fact that every tested cell line represents a different cancer type. Moreover, the culture conditions and the duration of exposure to hypoxia might have a serious impact, since the prolonged hypoxia and nutrient deprivation stress can alter the cellular fate by inducing quiescence to promote the reprogramming of cancer cells into a non-proliferating dormant state. Interestingly, previous reports have indicated that PC-3 spheres exhibit increased size and number when exposed to shorter durations of hypoxia (up to 72 hours) compared to normoxic conditions [59]. Similar findings were reported in experiments with the MDA-MB-231 cell line [60]. In prostate cancer cells, HIF-1α levels rise within six hours of hypoxia exposure and decline after 24 hours. Hypoxic stress triggers the demethylation of HIF-1α and HIF-2α, which in turn stabilizes NANOG mRNA, promoting a CSC phenotype [61]. Prolonged hypoxia pretreatment for 72 hours dramatically enhances primary and secondary mammosphere formation at one or two weeks after the termination of hypoxic exposure [62]. Our experiments involved continuous growth of tumor spheres under either normoxic or hypoxic conditions (1% O2) for the entire two-week period, yielding contrasting results. Based on our findings, we observed that long-term exposure to hypoxia led to the inhibition of tumor sphere formation in MDA-MB-231 and PC-3 cell lines. Furthermore, our results indicated a reduction in PCNA levels within tumor spheres derived from PC-3 and NCI-H460 cells.
CONCLUSION
To summarize, we propose that prolonged hypoxic conditions impede tumor sphere formation by prostate cancer stem cells, primarily through the downregulation of PCNA levels and the consequent suppression of DNA replication. Interestingly, in contrast to previous research, we did not observe any significant effect of chronic hypoxia on NANOG levels in tumorspheres across all five tested cell lines. Hence, the specific cellular responses to hypoxia appear to be influenced by the severity, duration, and other specific characteristics of the hypoxic stress. Consequently, personalized treatment strategies that consider individual patient characteristics, including intratumoral oxygenation, may offer improved and cost-effective approaches to prevent cancer metastasis.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ATP | = Adenosine triphosphate |
| CD133 | = Cluster of differentiation 133 |
| CD44 | = Cluster of differentiation 44 |
| CSC | = Cancer stem cells |
| DMEM | = Dulbecco's Modified Eagle Medium |
| DNA | = Deoxyribonucleic acid |
| EMT | = Epithelial-mesenchymal transition |
| HIF | = Hypoxia-inducible factor |
| HRP | = Horseradish peroxidase |
| KLF4 | = Krüppel-like factor 4 |
| mRNA | = Messenger ribonucleic acid |
| NANOG | = Homeobox protein NANOG |
| OCT4 | = Octamer-binding transcription factor 4 |
| PCNA | = Proliferating cell nuclear antigen |
| PMSF | = Phenylmethanesulfonyl fluoride |
| PVDF | = Polyvinylidene fluoride |
| RIPA | = Radioimmunoprecipitation assay |
| ROS | = Reactive oxygen species |
| RPMI-1640 | = Roswell Park Memorial Institute (RPMI)-1640 medium |
| SCM | = Sphere culture medium |
| SEM | = Standard error of the mean |
| SNAIL1 | = Zinc finger protein SNAI1 |
| Sox2 | = Sex determining region Y-box 2 |
| TBST | = Tris-buffered saline with Tween-20 |
| TERT | = Telomerase reverse transcriptase |
| TWIST1 | = Twist-related protein 1 |
| ZEB1 | = Zinc finger E-box-binding homeobox 1. |
AVAILABILITY OF DATA AND MATERIALS
All the necessary data and information are available within the article.
FUNDING
This paper has been supported by the Kazan Federal University Strategic Academic Leadership Program (Priority-2030).


